#NephJC Chat
Tuesday December 4 at 9 pm Eastern
Wednesday December 5 at 8 pm GMT, 12 noon Pacific
JAMA. 2018;320(19):2010-2019. doi:10.1001/jama.2018.15870
Effect of Lanreotide on Kidney Function in Patients With Autosomal Dominant Polycystic Kidney Disease
The DIPAK 1 Randomized Clinical Trial
Esther Meijer; Folkert W. Visser; Rene M. M. van Aerts; Charles J. Blijdorp; Niek F. Casteleijn; Hedwig M. A. D‘Agnolo; Shosha E. I. Dekker; Joost P. H. Drenth; Johan W. de Fijter; Maatje D. A. van Gastel; Tom J. Gevers; Marten A. Lantinga; Monique Losekoot; A. Lianne Messchendorp; Myrte K. Neijenhuis; Michelle J. Pena; Dorien J. M. Peters; Mahdi Salih; Darius Soonawala; Edwin M. Spithoven; Jack F. Wetzels; Robert Zietse; Ron T. Gansevoort; for the DIPAK-1 Investigators
PMID: 30422235 Full Text at JAMA
Introduction
Wait – didn’t we just have a #NephJC chat where we discussed a randomized, controlled trial (RCT) studying autosomal dominant polycystic kidney disease (ADPKD)? Kind of. About a year ago, we discussed the REPRISE study which found that the vasopressin antagonist, tolvaptan, slowed the loss of estimated glomerular filtration rate (eGFR) in patients with ADPKD and reduced eGFR. In October, we dove into the structure of the human PKD1-PKD2 complex, crucial to better understanding of ADPKD.
ADPKD is a disease of progressive cyst formation that can lead to rapid loss of kidney function once the eGFR begins to decline. Unfortunately, therapeutic options are limited to renin angiotensin-aldosterone system (RAAS) blockade, blood pressure control, and tolvaptan. The molecular biology of cyst formation growth is complex and may involve an increase in cyclic adenosine monophosphate (cAMP), mammalian target of rapamycin (mTOR) and Src pathways, and ciliary dysfunction.
Lanreotide is a somatostatin analogue that inhibits adenyl cyclase (the enzyme that produces cAMP) when bound to the somatostatin receptor – and thus should have an inhibitory effect on cyst formation and growth. Tolvaptan also inhibits cAMP. Somatostatin analogues, including octreotide and lanreotide have been studied in the past and successfully slowed cyst growth, though in small, short, and not always fully controlled studies. Specifically, the ALADIN trial suggested that lanreotide slowed cyst growth as measured by total kidney volume (TKV) at one year (though puzzlingly not at 3 years).
So, let’s discuss the RCT DIPAK1 (dipak means lamp in Sanskrit) that looked at the efficacy of the somatostatin analogue lanreotide in slowing the rate of decline in kidney function in patients with ADPKD.
The Study
Methods
Who’s In & Where/When Did They Come From?
18 – 60 years old
eGFR 30 – 60 cc/min/1.73 m^2
From 66 hospitals in the Netherlands
Who’s Out?
Bradycardia
Gallstones
Pancreatitis
“Diseases or medications that could confound the endpoint assessment” such as…
Diabetes mellitus
Use of non-steroidial anti-inflammatory drugs
Lithium or tolvaptan use
What did they get?
Block randomization into 2 groups (block size = 6) and stratification by:
age (older and younger than 45 years)
sex
eGFR (more than and less than 45 cc/min/1.73 m^2)
Group 1: Standard of care alone arm: BP < 140/90 mm Hg to be reached with Na-restricted diet + ARB or ACEi. Additional antihypertensive medications and dietary advice left to the discretion of the treating physician
Group 2: Treatment arm: standard of care plus 120 mg of lanreotide subcutaneously once every 4 weeks by nurses. The dose was lowered to 90 mg if the eGFR decreased below 30 mL/min/1.73 m2
Outcomes
Primary: Change in kidney function. The eGFR was calculated using creatinine and cystatin C
Secondary
Change in eGFR between pre and post-treatment visits
30% decrease from pretreatment eGFR or need for kidney replacement therapy
Change In htTKV between baseline and post-treatment visits
Change in health-related QOL between baseline and end of treatment visits
Adverse events and tolerability
Statistical plan
To show a 30% reduction in the eGFR slope with treatment, 150 patients would need to be enrolled in each group. The power calculation to get to this number assumed a 5 ml/min/1.73 m^2 loss of eGFR per year in the control group.
A mixed-model repeated measures analysis was used to evaluate the primary outcome and an exploratory mixed model repeated-measures analysis was used for secondary efficacy end points (change in eGFR, htTKV, and health-related QOL).
Funding Source
This study is supported by grants from the Dutch Kidney Foundation and the Dutch Ministry of Economic Affairs. In addition, IPSEN Farmaceutica BV (the manufacturer of the lanreotide preparation used in this trial), the Netherlands, provided an unrestricted grant. The funding bodies had no other role in study design, analysis or reporting.
Results
Between 2012 and 2014, 309 individuals were randomized to the treatment or control group (377 screened), with 23% and 8% drop-out rates in the treatment and control groups, respectively. No differences were noted in baseline demographics or patient characteristics (Figure 1, Table 1).
Mean duration of the treatment phase was approximately 26 months for the treatment group and 29 months for the control group. Lanreotide dose was decreased in 26 patients (in 14 patients because of eGFR decline). In patients on lanreotide, the mean administered dose was 112 mg.
At the end of the treatment, the mean difference in the slope of eGFR decline between both groups was not significant (p = 0.81, Figure 2).
Multiple pre-specified subgroup analyses (sex, age, baseline eGFR, htTKV, Mayo classification of disease, DNA class, urine volume) also did not show any statistically significant difference in the mean difference in the slope of eGFR decline (Figure 3).
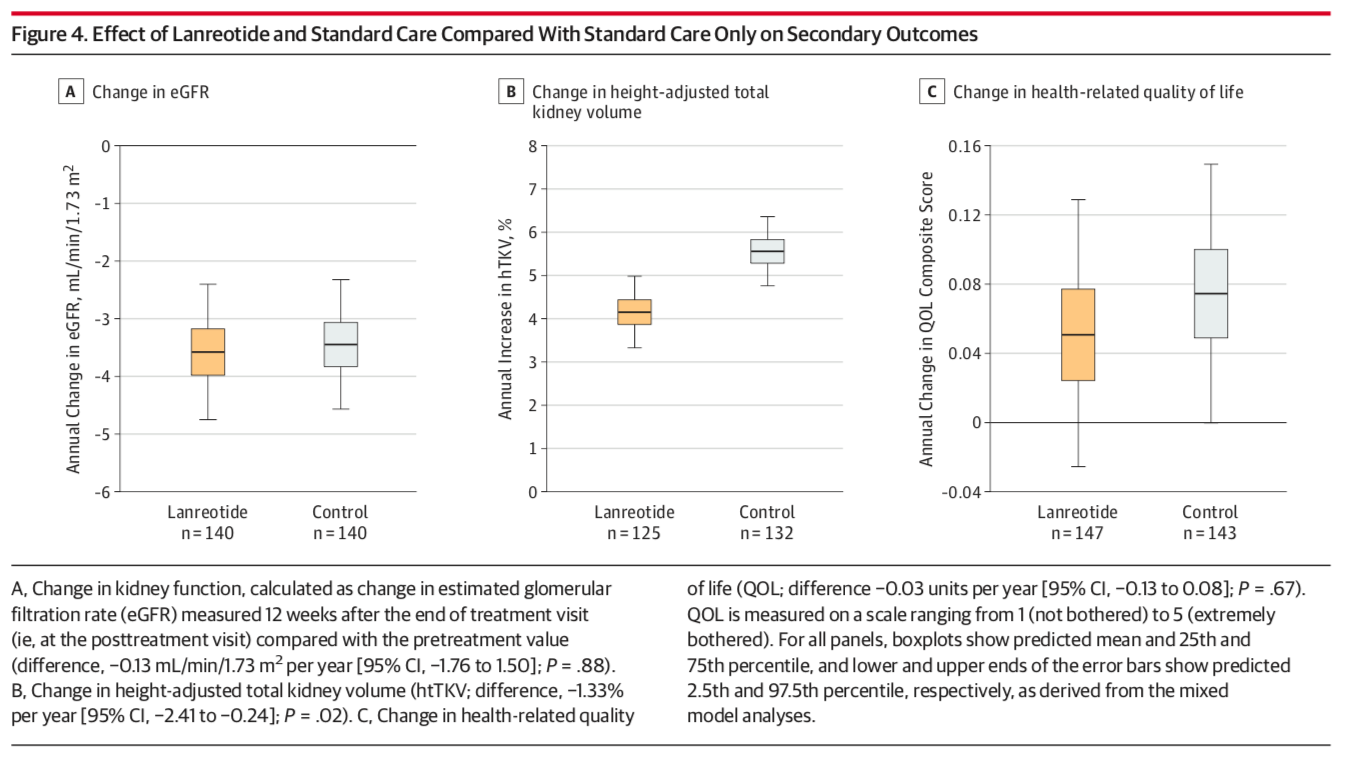
There was no significant difference in the change in eGFR or health-related QOL scores between pre and post-treatment visits, though the rate of change in htTKV was significantly lower in the lanreotide group (difference: -1.33%, p = 0.02, Figure 4).
The incidence of worsening kidney function was not statistically significant between the two groups (Figure 5).
Let’s not forget about adverse events – more hepatic cyst infections occurred in the lanreotide group as well as increases in glucose, hemoglobin A1C, and serum g-glutamyltransferase. There also seemed to be several other gastrointestinal symptoms experienced more often by the treatment group (Table 2).
Discussion
Take home messages: The use of lanreotide did not slow the decline in kidney function over 2.5 years of follow up, though it did slow the rate of TKV growth. The authors hypothesize that this slowing of growth may not have been significant enough to translate to functional benefit or that slowing cyst growth rate may simply not matter as much in those with advanced ADPKD. They also suggest that lanreotide may be nephrotoxic.
Some limitations of the study: This RCT was an open-label study (synthesis of a placebo compound to be injected subcutaneously was not possible) and enrolled a mainly white population. Second, the actual eGFR decline per year in the control group (-3.46 cc/min/1.73 m2) was lower than the power calculation assumption of -5.
Both the recently completed RCT ALADIN (75 patients, octreotide given for 3 years to patients with ADPKD and eGFR > 40) and DIPAK1 trials have now shown a beneficial effect of somatostatin analogues on cyst growth…but only ALADIN found a significant improvement in the rate of eGFR decline in the treatment group. To makes things even more confusing, tolvaptan in TEMPO 3:4 and REPRISE decreased both the rate of TKV growth and the decline of eGFR. Both vasopressin receptor antagonists and somatostatin analogues decrease cAMP – so why the conflicting results? Maybe we need a genie to help us with this one…
There are several ongoing trials in this area with this molecule though:
LIPS trial, in France, 156 participants with baseline eGFR between 30 to 89 ml/min
The ALADIN2 trial, with 100 participants with baseline eGFR 15 to 40 ml/min
TOOL: a small crossover trial of tolvaptan + octreotide
Share your thoughts and join us for #NephJC on Tuesday December 4 or Wednesday December 5! All are welcome, looking forward to seeing you there.
Summary by Samira Farouk
New York City, Mount Sinai Hospital,
NSMC Executive Board Member
Editor of Renal Fellow Network