September 4 and 5 we discuss kidney stones and antibiotics. How are the two related, you say? Join us. Find out more!
Atrial Fibrillation, Anticoagulation and Dialysis: Do We Finally Have A Better Option?
#NephJCBookClub next Tuesday and Wednesday
The fourth annual NephJC Summer Book Club is here and we are reading and discussing, Siddhartha Mukherjee's The Laws of Medicine, Field Notes from an Uncertain Science.
Water, water everywhere, but how much to drink?
RELIEF: More Fluids mean Less AKI?
Is Renal Denervation Innervated Again?
RADIANCE SOLO: Denervation with a Difference?
SPYRAL ON: Renal Denervation in Patients ON Medications
SPYRAL OFF: Can Renal Denervation Reduce Blood Pressure in Hypertension?
Renal Denervation: the Story so far...
The Sympathetic System and Blood Pressure
We know that the sympathetic nervous system plays a role in blood pressure regulation. A long long time ago, when we did not have many - or any - blood pressure lowering medications - especially safe ones, options considered were colloidal sulphur and sodium thiocyanate.
In the early years of hypertension, some thought that elevated blood pressure was a homeostatic response to decreased tissue perfusion. In renal failure, high blood pressure was supposed to be required to excrete larger amounts of urea despite fewer nephrons. See Ludwig Traube in 1856.
One of the few options to lower really high blood pressure was to perform a dorsolumbar sympathetectomy, first hypothesized by Brüning in 1923. After a few unsuccessful attempts, and some on serendipitious observations of the effects of spinal anesthesia, Adson did a bilateral ventral rhizotomy, from the T6 to L2 level in a young woman. Her blood pressure fell from 250/180 to 170/120 mm Hg. A subsequent case series, showed consistently lower blood pressure - truly & spectacularly so. The amazing improvement in blood pressure was accompanied by just-as-impressive side effects. Apart from surgical morbidity and occasional mortality, there was loss of sensation, paralytic ileus, problems with ejaculation, and loss of sweating. And then, a few years later, blood pressure lowering medications started becoming available, such as reserpine in 1949, and in the mid 60's Sir James Black developed propranolol. With the development of calcium channel blockers in the 1970s and captopril in the 1980s there was no looking back. Now A-C-D are established as first line therapy on the basis of safe and effective blood pressure lowering with beneficial effect on cardiovascular outcomes. The hot hypertension debates have moved on to what level of blood pressure lowering (120 or 130 or 140, not 250!) and not on use of surgical procedures and whether blood pressure should be lowered at all.
Renal Denervation Development
However, a minority of patients with hypertension have blood pressure that does not respond to first line agents (typically the A-C-D combination) mentioned above. Though second line agents are aplenty, they have higher rates of adverse events, as well as little data on long-term, hard-outcomes. Hence this area is still considered ripe for testing newer interventions, such as new drugs and novel strategies. One of the most promising entrants here was the development of percutaneous renal denervation, first reported in 2009, from Murray Esler, Markus Schlaich and colleagues from the Baker Institute in Melbourne. The patient they reported had severe resistant hypertension with end organ damage, underwent percutaneous radiofrequency ablation of the sympathetic nerves around both of the renal arteries, resulting in a decrease in kidney, as well as whole body norepinephrine spillover and muscle sympathetic activity. This was accompanied by an impressive 20/17 mm Hg drop in blood pressure - not as much as with dorsolumbar surgical sympathectomy, but impressive nevertheless given that this was on a background of multiple medications.
From Schlaich et al, NEJM 2009: the first case report in the modern era of RDN
The group quickly followed up with two large prospective trials. Symplicity HTN-1 was a single arm prospective trial, which enrolled 50 patients with resistant hypertension, defined as an office systolic BP of 160 or more on 3 or more drugs. It demonstrated a drop of 14/10 mm Hg at one month, which seemed to progressively increase to 27/17 by 6 months of follow up.
From Krum et al, Lancet 2009: Main result of Symplicity HTN-2
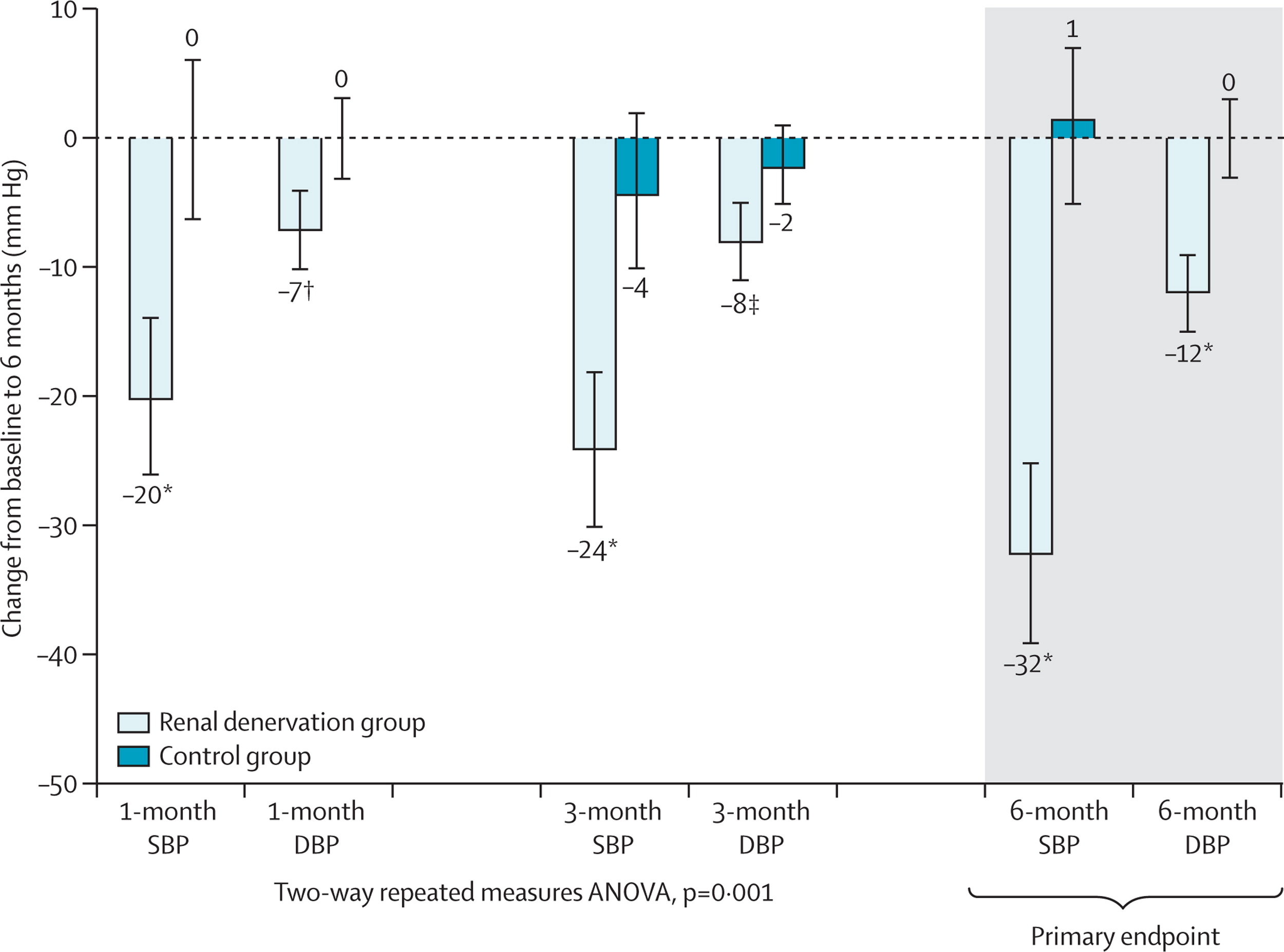
This proof-of-concept trial was followed by the Symplicity HTN-2, this was an open-label, randomized controlled trial in about 100 patients with resistant hypertension. The results showed a decrease in BP of 20/7 at 1 month and 32/12 at 6 months. Renal denervation (RDN) had arrived! CE approval in Europe followed soon after and rates of renal denervation soared. The company that developed RDN (Ardian) was bought by the device behemoth Medtronic for $800 million. Other device companies with different catheters entered the fray. Hypertension was cool again. Until Symplicity HTN-3.
From Esler et al, Lancet 2010: Main results of Symplicity HTN-2
The Crash
Despite CE approval in Europe, RDN was not approved in the US. The FDA insisted on a rigorous trial undertaken in the US. This was Symplicity HTN-3 (S-3). The catheter and technique were the same ones, now from Medtronic. But the trial was different in many salient ways. S-1 and S-2 included patients with an office BP > 160 mm Hg, and the outcomes were office based blood pressure changes. In S-3, the trialists not only required office BP > 160 on 3 or more drugs at maximal dosage, but also 2 weeks of home BP measurements followed by a repeat office visit to measure BP. They all also needed 24-hour ambulatory blood pressure monitoring (ABPM, see #NephMadness post on why this is important), showing daytime systolic BP > 135. This requirement resulted in the trialists enrolling 535 from 1441 potentially eligible patients. Most importantly perhaps, S-3 had a control arm in which the patient underwent renal angiography, with a ‘sham’ RDN procedure. Thus patients were blinded - and so were the trial personnel measuring blood pressure. The results of S-3 came as a shock to the hypertension community. They demonstrated no difference between RDN and control (ie sham RDN) in either office or ABPM. Many explanations were bandied about (see the emergency #NephJC session details and hangout video for more). But RDN was dead, as far as North America and many other places in the world were concerned.
From Bhatt et al, NEJM 2014, Main result of Symplicity HTN-3
Why did S-3 differ from previous RDN trials?
In the immediate aftermath of S-3, some of the key reasons that were thrown around were: heterogeneity of the population (there was a suggestion of less effect in the African-American population versus others) and the completeness of the RDN (the number of ‘notches’ corresponded loosely with the BP lowering). Medtronic developed a newer catheter system (‘Spyral’) which could do radioablation at different points both longitudinally and circumferentially, thus reducing the issue of operator expertise.
Design of the newer SPYRAL catheter system
With respect to the efficacy of actual nerve ablation, it is indeed important to note that there is, yet, no physiological test or biomarker to assess whether the RDN was successful. The norepinephrine spillover or muscle sympathetic nerve activity testing are accurate, but time consuming and only used as research tools. Blood pressure lowering is a physiological test, but it is very noisy.
On the noisiness of blood pressure, one of the fascinating analysis that came from S-3 was from Darrel Francis’s group (yes the same Professor of ‘tweetorial’ fame). A few months before S-3, they predicted that the effect in a sham-controlled trial would be less than in previous trials. S-3, of course, didn’t show just a smaller effect, it reported no effect of RDN compared to sham. So Francis and his smart fellows (James Howard) conducted a further quantification of the three biases that they measured as being important to understand in this area.
Regression to the mean: First described by Francis Galton (‘regression to mediocrity’) this is an important aspect of a messy measurement like blood pressure. Selecting individuals with very high values and repeating another measure (as was done in the early RDN studies) is almost guaranteed to provide lower values - whether patients undergo RDN or not. Repeated measures, to confirm that the elevated blood pressure is truly elevated (say, with 2 weeks of home BP, or with ABPM), help reduce this - and a control group almost solves this. However, in the RDN studies, there was not much of a difference between controlled and uncontrolled studies - and even with unblinded controls who were randomized.
The second important bias then is information bias: in this case measurement bias. Observers who were unblinded taking BP measurements can be, ahem, biased, if they know whether the patient had or did not have RDN. Hence the use of automated measures (such as ABPM) reduces the effect size.
Lastly, blinding - which was a key aspect that was different between the two RCTs (S-2 and S-3). How would this have a different effect compared to unblinded controls? After all, BP - especially with ABPM, is an objective measure? However, knowing that one has had RDN may change behaviour - maybe patients become more adherent to their medication. Perhaps even the elusive placebo effect does truly exist in hypertension.
From Howard et al, Circulation 2016. Relationship between trial design and the reductions in office and ambulatory blood pressures. Each data point represents a trial. The area of the data point is proportional to the trial size. Red diamonds indicate the meta-analyzed estimate of the effect size for each trial design
Apart from these three aspects, it was also noted - from the same group - that variance in BP trials was higher when greater number of blood pressure medications were prescribed.
From Howard et al, J Hypertension 2015. Visit-to-visit variance in blood pressure within individuals increases with the number of medications prescribed. Sample size required for a trial is linearly dependent on visit-to-visit variance, the square of the visit-to-visit SDΔ, because the sample size required for a trial is linearly dependent on this.
Among the recommendations provided to overcome this problem were:
Solution 1: conduct renal denervation trials in patients on no medications (note this is exactly opposite of what was being done so far: testing RDN in patients with resistant hypertension)
Solution 2: continue prior medications, but pause medication for a limited period prior to baseline and final pressure measurements
Solution 3: directly observed antihypertensive therapy with a stable regimen
Based on this, in late 2014, a think tank comprised of representatives from the FDA, along with the NHLBI and the American Society of Hypertension came together and published some guidance for device therapy trials in hypertension, which was followed closely in the three trials we will be discussing at #NephJC this week.
Read so far? Now please go ahead and check out summaries of
Also check out the excellent Visual Abstracts from Angel Ortiz
Lastly, to prepare for the chat, see the summary of all three trials - and some discussion points.
Post by Swapnil Hiremath, Ottawa
#NephJC next week: Renal Denervation Bonanza
Growing Organoids in a Dish
Donating Organs & Drug Overdoses
Tonight, join us for an #AskASN discussion of belatacept in transplant
Tuesday, May 15 at 9pm EDT #AskASN returns for a discussion of belatacept with transplant experts Matthew Ellis, MD from Duke and Silvi Shah from Cincinnati.
Here is a visual abstract by Aakash Shingada on the BENEFIT trials to wet your appetite.
Nephrocystin: More Notable than we thought?
To most Nephrologists, Nephronophthisis (NPH) is considered to be a rare autosomal recessive paediatric disease. However, this article by Snoek et al challenges that belief, by examining the prevalence of mutations that cause NPH, in adults with end stage renal disease (ESRD). Read our NephJC summary below and come along to our Twitter Journal Club discussion to find out whether you might consider a diagnosis of NPH, next time you see that patient carrying a label of ‘ESRD, cause unknown’.