#NephJC chat
Tuesday Nov 29th 9 pm Eastern
Wednesday Nov 30th 8 pm GMT, 12 noon Pacific
Lancet. 2016 Nov 18. pii: S0140-6736(16)32187-0. doi: 10.1016/S0140-6736(16)32187-0. [Epub ahead of print]
Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open-label, multicentre, randomised controlled trial.
Thomusch O, Wiesener M, Opgenoorth M, Pascher A, Woitas RP, Witzke O, Jaenigen B, Rentsch M, Wolters H, Rath T, Cingöz T, Benck U, Banas B, Hugo C.
PMID: 27871759
Link to Editorial in Lancet (gated)
Additional background reading: RFN post by Paul Phelan (rATG vs Basiliximab #DreamRCT)
Background
Over 55% of kidney transplant centers across the USA, use rATG (rabbit anti-thymoglobulin or Thymoglobulin) as the induction agent of choice, despite the fact that it is only FDA-approved for the treatment of acute cellular rejection. rATG is composed of polyclonal antibodies that target multiple T cell antigens, although it may also have activity against B cells, monocytes and neutrophils. It is quite expensive.
One alternative induction agent is Basiliximab (Simulect), a humanized anti-CD25 monoclonal antibody that targets the alpha-subunit of the IL-2 receptor (CD25), which is critical for T-cell activation. Basiliximab is less expensive than rATG. Here is a good, open, review of these two induction agents and other immunosupression (IS) medications.
The results of the landmark RCT, ELITE-Symphony trial (WikiJournal Club review) revealed that low-dose tacrolimus, mycophenolate mofetil (MMF) and steroids along with an IL-2 receptor antagonist (daclizumab) induction agent, has better efficacy than cyclosporin (at standard or low dose) or sirolimus. However, the low-dose tacrolimus arm showed more new onset diabetes after transplant (NODAT). Since its publication, Elite-Symphony has guided the standard of care for maintenance IS.
Since ELITE-Symphony many centers in the USA have experimented with steroid-free maintenance IS protocols. There is observational data showing that corticosteroids are associated with death with functioning grafts caused by cardiovascular disease or serious infections during the first 2-5 years following transplantation. Pushing against steroid-free IS is data showing increased rate of T-cell mediated rejection (TCMR), which ironically would be treated with more steroids.
Methods
Aim of the study: To assess which of the two induction agents (rATG or basiliximab) was most efficacious at permitting rapid steroid withdrawal in tacrolimus and MMF-based IS therapy within the first year, compared to basiliximab with standard maintenance steroid regimen.
Study Design and methods: 21 centers in Germany, prospective, RCT, open label, three arms. Patients were randomized to 1:1:1
- Arm A: Basiliximab as induction + maintenance steroids (controlled arm)
- Arm B: Basiliximab as induction and rapid steroid withdrawal
- Arm C: rATG as induction and rapid steroid withdrawal
Primary end-point: Biopsy proven acute rejection (BPAR) at one year (as per Banff criteria of 2005)
Secondary end-points: Patient and graft survival, graft function, incidence of NODAT, Systolic and diastolic blood pressure, lipids (HDL, LDL and triglycerides), bodyweight, post-transplant infections, incidence of malignancy, wound healing disorders, cataracts and osteoporosis.
Inclusion criteria: Ages 18-75, low immunological risk, deceased or living kidney transplant and second renal transplant (with some limitations), ABO compatible, negative crossmatch (CDC-test), PRA less than 30%,
Sample Size: The study set out to prove that rATG (arm C) would be superior to both basliximab arms (arm A and B). The BPAR rates were expected to be 6.4% in arm C and 17% in arms A and B. Thus it is a trial powered to show that rATG is superior, rather than that steroid withdrawal is safer.
Funding: The study was funded via Astellas, Roche and Sanofi: however the study design, conduct and analysis was done by the investigators themselves.
Results:
Of the roughly 600 patients randomized into the study (~ 200 in each arm), all of whom were analysed in the intention-to- treat analysis, 473 made it to the full 12 month follow up (and per protocol analysis)
Figure 1 from Thomusch et al, Lancet 2016
Table 1 from Thomusch et al, Lancet 2016
Figure 2 from Thomusch et al, Lancet 2016
Rabbit ATG did not show superiority over basiliximab induction for the prevention of BPAR after rapid steroid withdrawal within 1 year after renal transplantation (as was the assumption of the sample size calculation). The BPAR rates in arms A and B (basiliximab with or without early steroid withdrawal) were also similar.
There were a host of secondary outcomes (see table 2 from paper) however, the one of interest is presented below:
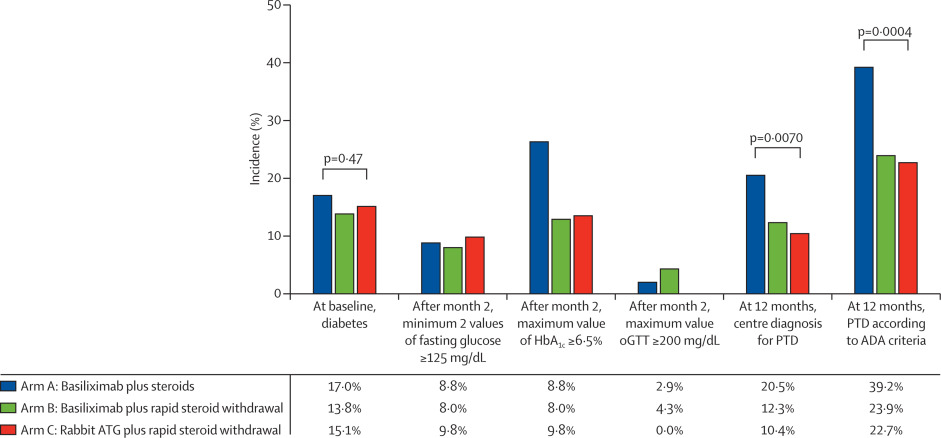
Figure 3 from Thomusch et al, Lancet 2016
To put this increase in NODAT with no steroid withdrawal, lets compare all relevant outcomes together:
Limitations:
The authors acknowledged that the study was underpowered, was not blinded, had a short follow-up period and lacked racial and ethnic diversity. Other limitations I observed included: protocol biopsies were not mandatory, a wide range of tacrolimus trough levels, and uncertainty whether a renal pathologist with experience in kidney transplantation was the person reviewing the biopsies.
Author Conclusions:
“rATG did not reduce the incidence of BPAR compared to basiliximab with or without corticosteroids, both induction agents proved to be equally effective in steroid-free maintenance therapy… with substantial benefits in regard to post-tranplantation diabetes”
My take home points:
If you consider how the sample size was calculated, strictly speaking, the result is more along the lines of: they failed to show that rATG with early steroid withdrawal is superior to basiliximab (with or without early steroid withdrawal) for preventing BPAR. This study was not powered to show non-inferiority or equivalence between the three regimens.
Although the follow-period was short (12 months), data revealed good allograft function, less rejection (BPAR) and less post-transplant diabetes in patients who underwent early steroid withdrawal. The authors are planning to publish the 5-year follow-up soon. Something we would like to know is what is the long-term graft and patient survival. It is Important to point out the use of once-daily extended release tacrolimus in this study as compared to twice-daily standard release, which appears to be more common practice, although evidence has shown similar outcomes (here & here).
Another point I want to address is the wide range of the blood concentration of tacrolimus levels; although I don’t think targeting a narrowed target would have changed outcomes, maintaining levels within a narrow target range can be challenging.
Although some of the results of this study are not surprising (e.g. steroids are associated with multiple cardiovascular events and diabetes), it shows the similar outcomes between two induction agents: basiliximab and rATG. Some transplant experts think that basiliximab is a “very weak” immunosuppressant; however this trial indicated similarities to rATG and it’s a reasonable alternative for induction in kidney transplantation. It’s less expensive in comparison to rATG and it’s important to consider in patients with low immunological risk. In conclusion, HARMONY may be better than SYMPHONY.
Summary by Hector Madariaga, Nephrologist, Boston
#NephJC chat
Tuesday Nov 29th 9 pm Eastern
Wednesday Nov 30th 8 pm GMT, 12 noon Pacific







Fantastic chat. Lot of participation - though the study didn't seem to move the needle much!