Diabetes mellitus roughly translates as "sweet urine" but in this era of sub-7% glycated hemoglobins the name has lost it's meaning. But with the new SGLT-2 inhibitors well controlled patients with diabetes are once again making sweet urine and additionally avoiding cardiovascular outcomes and apparently are effectively battling diabetic kidney disease.
Diabetic kidney disease is one of the most important diseases in nephrology. Alone, it accounts for 46% of incident dialysis in the United States.
We have a limited set of tools to alter the outcomes of diabetic kidney disease. We can control the blood sugar, control the blood pressure, and use ACEi or ARB. And that is about it. That is all we have, and we have had those since the early 90's. In the decades since, we have not had anything survive the sharp knives of prospective randomized controlled trials. That is until last November when EMPA-REG investigators revealed the renal results from their cardiovascular trial at Kidney Week 2015.
EMPA-REG was a post marketing study demanded by the FDA to look at cardiovascular outcomes for patients taking the SGLT-2 inhibitor, empagliflozin, an oral hypoglycemic medication. The FDA is requiring all new oral diabetic drugs to prove that they do not worsen cardiovascular disease. The first four of these studies were all essentially unremarkable, without showing increased heart trouble but also not showing any CV benefit. EMPA-REG changed all that by showing improved CV survival in the patients taking empagliflozen.
Empagliflozin is an SGLT-2 inhibitor. This prevents the kidney from reabsorbing much of the filtered glucose in the proximal tubule, resulting in glucosuria. Normally all of the glucose one eats is readily metabolized. With SGLT-2 inhibition provides a second fate for ingested glucose, the toilet bowl. By blocking glucose in the proximal tubule it is a form of drug induced Fanconi syndrome similar to acetazolamide.
EMPA-REG randomized 7020 patients to either placebo, 10 mg or 25 mg of emagliflozen. The study was powered to show non-inferiority in terms of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. This composite outcome occurred in 10.5% of the empagliflozen patients and 12.1% of the placebo patients after a mean of 3.1 years.
The trial enrolled patients with diabetes type 2 (duh) and pre-existing cardiovascular disease. The study's prespecified statistical plan included a renal outcome they called incident or worsening nephropathy:
Of note, 11% of the patients started the trial with macroalbuminuria so they were excluded from this end-point. Otherwise it is a pretty good composit outcome, though development of macroalbuminuria is not patient oriented. My patients don't want to end up on dialysis, they are not that concerned about crossing an imaginary line in the sand with regards to the amount of protein their kidneys spill.
The authors added two post-hoc end-points:
The first limited their analysis to patients with CKD stage 3 disease and the second was a modification of the primary renal outcome but excluded the proteinuria outcome. This importantly is also almost identical to the outcome used in the RENAAL and INDNT studies that made ARBs standard of care in patients with type two diabetes and diabetic kidney disease:
The principle difference is that EMPA-REG used renal death and the ARB landmark trials both used total deaths. I wonder what the reason was for this subtle difference. I also assume that these post-hoc end-points include all patients, rather than excluding the 11% with pre-existing macroalbuminuria.
One note about the outcomes, the eGFR at study enrollment was done by MDRD, but during the study they used CKD-EPI. I doubt this would make much of a difference, but shouldn't you go home with the girl you brought to the dance?
The authors used a "modified intention-to-treat approach to perform analyses in patients who had received at least one dose of a study drug." Modifying intention to treat makes the hair on the back of my neck stand-up but I doubt it affected the outcomes here, as it looks like this "modification" only left out 8 patients (7028 randomized, 7020 analyzed).
Results
This was a massive, international study:
There was a pre-specified microvascular outcome:
first occurrence of any of the following: the initiation of retinal photocoagula- tion, vitreous hemorrhage, diabetes-related blind- ness, or incident or worsening nephropathy.
This outcome showed a 38% relative risk reduction with epagliflozen but the difference was entirely driven by the renal outcomes.
Figure 1 in the trial is a Kaplan-Meier plot of the primary renal outcome and the post-hoc analysis excluding proteinuria. Both show impressive renal protection:
Each component of the primary renal outcome was significantly reduced by empagliflozen. However new microalbuminuria was not significantly reduced by empagliflozen, 51.5% with treatment and 51.2% with placebo.
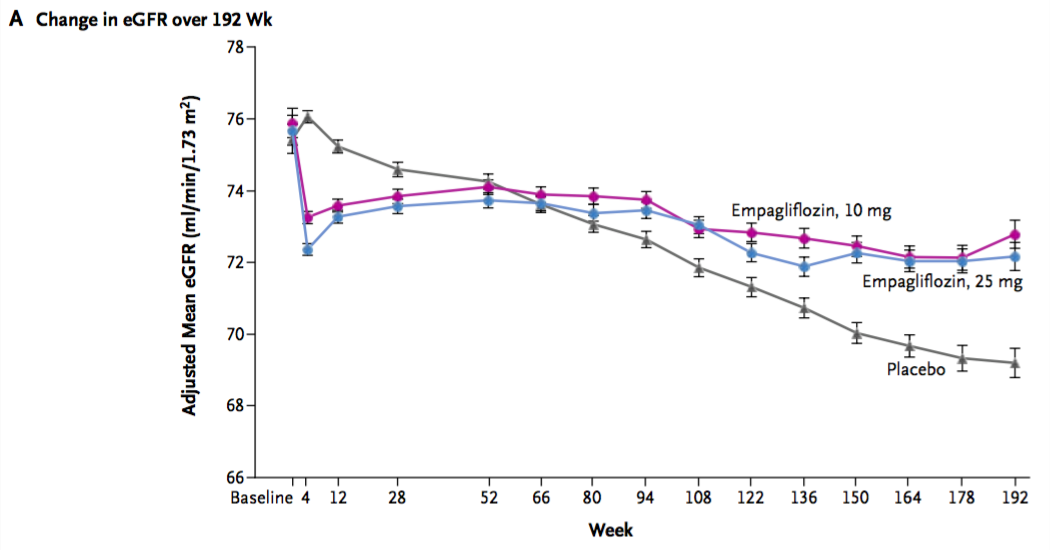
The investigators also provided data on progression of renal failure. This is of particular interest since empagliflozen was not licensed for use in advanced CKD due to data showing significant reductions in GFR. However these phase three trials were only 52 weeks in duration. Use of empagliflozen causes an acute drop in GFR which then appeared to recover over the subsequent 52 weeks but it didn't quite make it back to baseline:
Table is from the Jardiance package insert.
The EMPA-REG data shows a similar acute drop in GFR but then it subsequently partially recovered and then remained stable through the remainder of the study while the control group had a constant gradual decline over three years:
Here's how the authors characterized the change in GFR during the study:
the eGFR remained stable in the empagliflozin groups and declined steadily in the placebo group, with adjusted estimates of annual decreases of 0.19±0.11 ml per minute per 1.73 m2 in the 10-mg and 25-mg empagliflozin groups, as compared with a decrease of 1.67±0.13 ml per minute per 1.73 m2 in the placebo group...
After the study concluded, all study drugs were stopped and patients returned for a final eGFR assessment. Patients in the emagliflozen groups returned to their original baseline eGFRs while the palcebo patients had no similar recovery. This indicates that the early loss of eGFR with empagliflozen is entirely hemodynamic, analogous to the drop in GFR seen with ACEi.
Adverse events
The only adverse events more common with empagliflozen was genital infections. There was no significant increase in UTI, complicated UTI or pyelonephritis with SGLT-2 treatment.
Urosepsis was uncommon but more likely with empagliflozen.
Hypoglycemia, diabetic ketoacidosis, thromboembolic events, bone fractures, and volume depletion were not more common with empagliflozin.
Hyperkalemia was more common with placebo.
Acute kidney injury was more common with placebo.
Discussion
This empagliflozin victory was largely an unexpected finding. And the renal protection is a secondary afterthought of an endpoint in a trial designed to look for cardiovascular events. Of course, I would love to see a study specifically designed to find a benefit in renal outcomes. But this is an important finding and it demonstrates a relative risk reduction for renal outcomes roughly twice as strong as found in RENAAL and IDNT. It is hard to look at these findings and do anything but whoop and holler.
I used the post-hoc EMPA-REG renal outcome, as that was most similar to the primary outcomes in the ARB trials.
I look forward to additional data, especially from the other SGLT-2 inhibitors. Do they also provide renal protection? Demonstrating that this is a replicable, class-effect will be an important step in validating these results.
Another important question, is that now that we have good data that the drop in eGFR is hemodynamic, temporary and analogous to the changes seen with ACEi and ARBs, can we use these agents in patients with GFRs south of 30 ml/min? This will require empiric data but having an agent that provides renal protection while reducing hyperkalemia would be like a pharmacologic angel for many patients.