#NephTrials Chat
Tuesday May 25th 9 pm Eastern
In the first edition of #Nephtrials we used the Phosphate Trial, an ongoing pragmatic trial examining optimal phosphate targets in dialysis patients as a lens to understand pragmatic trials. Read more about it here.
We now zoom in on a specific kind of pragmatic trial design: the cluster randomized trial (C-RCT) and try to decipher the ways in which this design may be uniquely suited for conducting clinical trials in dialysis patients.
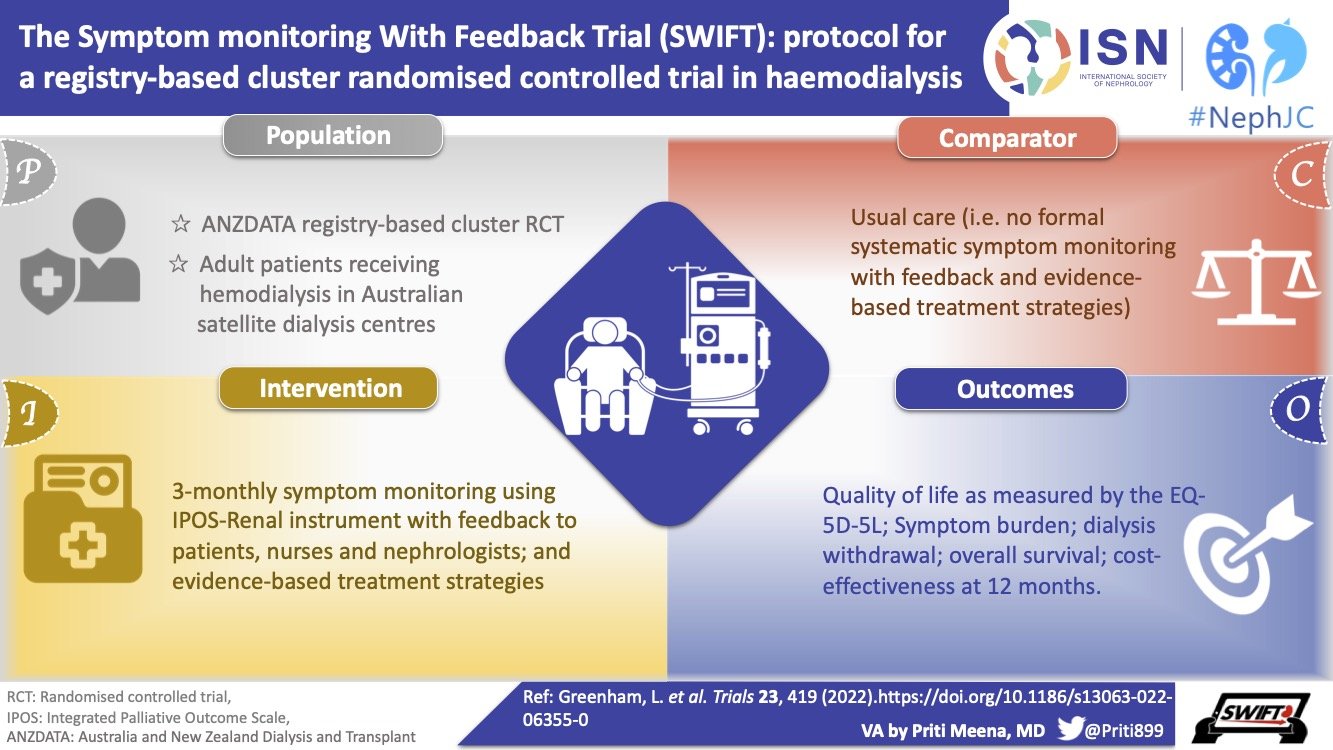
We will focus on DIAL-MAG CANADA - a trial designed to compare outcomes between patients on higher vs lower dialysate magnesium. More about the trial here. An interesting secondary outcome measure is the effect of higher dialysate magnesium on patient reported muscle cramps- a major and poorly understood symptom, heavily affecting patients’ quality of life.
What are Cluster RCTs?
We know that pragmatic trials are designed with the intention to inform a clinical or policy decision by providing evidence for adoption of the intervention into real-world clinical practice.
Cluster randomization, which involves randomly assigning groups of patients to to an intervention or comparator, is popular in pragmatic trials. A pragmatic approach is easier when an intervention is implemented at a group level rather than at an individual level — this is one of the reasons why pragmatic trials commonly incorporate cluster randomization. These trials often evaluate interventions that are already in use in clinical practice and allow for broader inclusion criteria, easier involvement of physicians delivering the intervention, and recruitment of patients, routine clinical participant monitoring, and follow-up.
Are Cluster RCTs the same as Pragmatic RCTs?
Most, but not all cluster randomized trials are pragmatic RCTs. Clusters can constitute clinics, schools, villages etc. These trial designs are used when individual randomization is either logistically impractical or not feasible.
Some excellent recent examples of Cluster RCTs are the two studies which compared the use of isotonic saline with that of balanced crystalloid solutions- SALTED and SMART. See our coverage on these two trials at #NephJC and also the accompanying #Nephstats commentary on pragmatic trials.
What is a cluster?
The unit of randomization in C-RCT is a cluster, which could be a physically discrete unit, or location, or a discrete amount of time. A key assumption in traditional randomized trials is independence, which means that the care of one participant in the trial does not alter the care of others. However, in trials that affect many people at once, including any time you intervene on an entire clinic at once, that assumption is not met. Clustering addresses this problem.
In possibly the largest trial ever done, (~ 1 million participants) Deworming and Enhanced Vitamin A supplementation, (DEVTA, Lancet 2013) entire villages were a cluster with the intervention (vitamin A or deworming) delivered via healthcare centres. In the SMART and SALT-ED trials, the cluster was a month, with alternate months randomized to balanced versus saline fluids. In the examples we will be discussing today, the clusters are dialysis units. However, since a cluster is a large number of participants, how do you consent everyone? Do you even consent everyone, or anyone?
How do you consent in a cluster RCT?
It's simple and it's complicated. It depends. The simple answer is that one need not always require consenting at all. But sometimes you do. After all, one could not consent a million people from the example above. But if something is not feasible, is that enough from an ethical point of view? This flowchart walks through some of those principles:
Figure from Sim et al, AJPH 2012
Cluster RCTs can be broadly thought to be individual-cluster or cluster-cluster. For example, in the Hi-Lo trial (Edmonston et al, AJKD 2021), the intervention (serum phosphate target) is focused on individual patients. So, even if a dialysis unit is included and randomized, an individual patient may or may not be included depending on their willingness to participate.
Figure from Taljaard et al, BMJ 2013
On the other hand, if the intervention is focused on the health providers or team, as in this cluster RCT (Tangri et al, JASN 2021), where the intervention was an educational intervention, using knowledge-translation tools, including telephone survey, a center-specific audit with feedback, and delivery of a guidelines package, individual patient consent does not make sense.
Figure from Taljaard et al, BMJ 2013
The Ottawa Statement (Weijer et al, PLoS Medicine, 2012) explains an ethical framework (see list below) covering these and many other aspects for the interested reader.
Even if consent is not obtained or necessary, there are other crucial aspects such as the role and oversight of ‘gatekeepers’ (e.g. the institutional review boards) and the provision of information to participants, and sometimes an option to ‘opt out’.
Needless to say, this is a topic which has to be carefully thought out and dealt with. Additional reading for the interested reader:
McRae et al, ‘When is informed consent required in cluster randomized trials in health research?’ Trials 2011
Weijer et al, ‘The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials’ PLoS Medicine 2012
Sim et al, ‘Informed Consent and Cluster-Randomized Trials’ Am J Public Health 2012
What is contamination and why is it a bigger threat in a Cluster RCT?
Contamination can occur when individuals in the control arm are partially exposed to the intervention through interaction with individuals receiving the intervention. This is more common in behavioral interventions eg: dietary interventions or education regarding hand washing practices to reduce infections, in which the control group could get exposed and start adopting these interventions. When contamination occurs during a clinical trial, it will dilute the observed differences between comparators and can affect the reliability and validity of the study.
On the other hand, lack of uptake of intervention is also problematic. In the TIME trial (Dember et al, JASN 2019), dialysis facilities were randomized to the intervention and adopted a default session duration of ≥4.25 hours (255 minutes) for patients initiating maintenance hemodialysis. Dialysis facilities randomized to usual care had no trial-driven approach to session duration.
The TIME trial was terminated early because of a lower than anticipated difference in session duration between the intervention and usual care groups.
X axis represents the number of participants who were dialyzed for more than 4.25 hours. Intervention was actually delivered for the majority of sessions for a very small proportion of patients [C] in comparison to those who were prescribed it [A]. So Intervention should have been all dark blue, and Usual care all red. The colorful panorama represents crossover or contamination.
For individual participants in both groups, session durations decreased as months of dialysis care increased, but this effect was greater for intervention than for usual care patients. Per authors, discussions with facility staff and medical directors during the course of the trial indicated that the major reasons for poor uptake of the intervention were unwillingness by patients to have longer dialysis treatments, perception by the treating nephrologists that longer dialysis was not needed because of adequate solute clearance, and perception by the treating nephrologists that longer session durations were not in the best interest of a patient because of older age and/or frailty. Despite an appreciation by participating nephrologists for the major hypothesized benefit of longer sessions, changing an ingrained practice for the purposes of research proved to be difficult. So it’s not surprising that no effect was seen. TIME demonstrated that a cluster RCT can be performed in US dialysis units, but it did not inform us about the efficacy of longer dialysis and clinical outcomes. This is an issue if the intervention cannot be rolled out in a cluster RCT. A pragmatic take also can be: even if we find out that longer dialysis improves clinical outcomes, TIME demonstrated that it will be difficult to roll out longer dialysis within contemporary practice patterns in the US.
What is a Stepped wedge design in a cluster RCT? What are other ways to think of Randomization in a cluster RCT?
Simple randomization can sometimes lead to imbalances between the trial arms. Several methods of randomization are available to balance the confounding factors.
In certain studies, exposing only some clusters to intervention may not be ethical or preparing all of the intervention clusters to start the intervention at the same time may not be feasible. The stepped-wedge CRT design overcomes this problem by gradually introducing the intervention to groups of clusters over time. Clusters are divided into several groups, usually 4 to 6, and all clusters start the trial in the control condition. Groups of clusters cross over to the intervention condition in random order and on a staggered schedule.
With pair matching, clusters are paired in terms of their potential confounders and then within each pair, one cluster is randomized to receive one of the arms and the other cluster receives the opposite arm. In stratified designs, the investigators choose the factors that they want to balance between the different arms of trials and the trial population is divided into subgroups called strata. Then within each stratum, a randomization scheme that ensures balance is developed.
Image courtesy https://www.scribbr.com/methodology/stratified-sampling/
Constrained randomization is a method used when simple randomization cannot be relied upon to balance key group-level prognostic factors across the comparison arms due to the availability of only a small number of groups. Clusters are characterized in terms of several potential confounders. Different randomization schemes are generated and tabulated and are given balance scores. By some predefined criterion, such as a certain percentage of all possible randomizations, a set of clusters with the least amount of imbalance is chosen as the “randomization space.” From this “randomization space,” a single randomization scheme is selected
What kind of biases can still occur with cluster RCTs?
Allocation concealment is regarded as crucial for individually randomised trials so that bias in the patients recruited can be avoided. But recruiting subjects blind to their allocation status is very challenging in C-RCTs.
Selection bias can occur when the study population systematically differs from the target population leading to errors in association or measured outcomes. In C-RCT, selection bias can occur at individual or cluster level as randomization frequently precedes recruitment or consent.
C-RCTs in which the randomized clusters are aware of being in the intervention arm can introduce recruitment bias as participation can become more attractive in the intervention arm. For example, consider a hypothetical C-RCT in which CKD clinics are identified as clusters and are randomized into 2 groups- one group has an in house dietician for dietary counseling (intervention) vs the 2nd group in which patients are sent home with reading resources (control) but not given in person counseling, thereby making the first group more favorable for referring primary care providers and for patients. This may lead to more recruitment in the intervention arm resulting in recruitment or selection bias.
What is the sample size in a cluster RCT?
Determining sample size in cluster RCT can be challenging but remains a critical aspect of trial design. A cluster RCT seems to have two sample sizes. The first is the cluster, like the number of clinics randomized. The second are the individual participants, the number of patients in each of those clusters. We can recognize that adding more clinics will clearly add more sample size to the study, but so can adding more participants to each clinic. In this way the power and sample size of a cluster RCT is between that of the cluster and the individuals that constitute the cluster. How far between? That actually depends on details of the trial.
We mentioned the DEVTA trial above with a million participants. However, a million participants were not actually randomized, the randomization units were 72 administrative blocks (36 in each arm). Patients within the same cluster are more likely to have similar outcomes for many reasons (quantitatively referred to as the intra-cluster coefficient or ICC), and hence cannot be regarded as independent causing the effect estimate to be less precise and with wider confidence intervals. As an extreme example, a trial with only two clusters of 500 patients each will have little power compared to a trial with 10 clusters of 50 patients each. See the table and figure below (from Hemming et al, BMJ 2017) for actual examples.
In the examples worked out above, the ICC was assumed to be constant at 0.03. The ICC is not constant at all though, and depends on the trial setting and the intervention, among other factors, stressing the need to have cluster trial experts involved in the design and analysis. More on that below.
How are cluster RCTs analysed?
One way is to conduct the analysis at the same level as the allocation, using a summary measurement from each cluster. Then the sample size is the number of clusters and analysis proceeds as if the trial was individually randomized (though the clusters become the individuals). However, this reduces the power of the study, depending on the number and size of the clusters! So how do we count individual patient data when clusters were randomized? Such an analysis might be based on a ‘multilevel model’, a ‘variance components analysis’ or may use ‘generalized estimating equations (GEEs)’, among other techniques. It has to account for the intra-cluster coefficient we mention above. Additionally, these analytic methods can also account for covariates - at an individual patient level - as well as the cluster level in a step-by-step basis. Needless to say - these are not procedures for the faint-hearted, and expert trial statistician involvement is necessary.
How should we interpret the results of cluster RCTs?
Again the answer is, it depends. One has to keep in mind the actual study design, the unit of randomization and the intervention. For example, in a cluster RCT (Manns et al, cJASN 2012), primary care practices were randomized to usual practice or enhanced prompts, including recommendations in patients with GFR < 60 to measure urine albumin/creatinine ratio, prescribe an ACEi or ARB in patients with diabetes or albumin/creatinine ratio >35 mg/mmol, reduce BP to <130/80 mmHg, reduce LDL cholesterol to <2.5 mmol/L, and target hemoglobin A1C to <7% in patients with diabetes. We know that ACEi/ARB decrease kidney failure in this population - but this trial was negative! So the interpretation is that in elderly patients with CKD and an indication for ACEi/ARB, an enhanced laboratory prompt did not increase use of these medications. Similarly, an electronic clinical decision support system with or without pharmacist follow-up (Peralta et al, AJKD 2020) did not result in an improvement in blood pressure - though we know that medications do lower BP! This trial tells us specifically that deploying a clinical decision support system did not decrease BP compared to usual care.
Despite the challenges with design, analysis, interpretation, and ethical issues in C-RCTs, specifically in the field of Nephrology, the dialysis units are well suited for pragmatic cluster RCTs because of standardized approaches to treatment and data acquisition, and a well-developed infrastructure for delivering care to patients.
Why are dialysis units suited for performing pragmatic cluster trials?
Study interventions within the range of standard practice, can be adopted as local policy as in real-world dialysis settings in which many aspects of care are provided without disclosure - eg dose of medications, dialysis temperature etc.
If all the patients within a dialysis center are randomized as a cluster, it helps prevent a perception among the patients that they are being deprived of the benefit of intervention and can help perform the study incorporating even the most vulnerable patients with serious comorbidities, advanced dementia, and frailty.
Since patients in the dialysis unit are in close proximity to each other, this can cause contamination in a traditional RCT setting, and hence a cluster RCT is better suited in this situation.
Delivery of intervention in this setting requires involvement of a multidisciplinary team including physicians, dietitians, social workers, nurses, and technicians and hence following a single protocol for the cluster as a whole is more practical. Individual randomization in such a setting may pose many hurdles in adherence to protocol and may not be feasible.
A cluster RCT design can allow involvement of investigators from community including non-academic private practices and community level nephrologists as opposed to traditional RCTs which are usually designed and implemented in bigger academic centers.
Cory Goldstein and colleagues have elaborated much more on ethical issues in pragmatic C-RCTS in dialysis facilities, especially the lessons from the TIME trial in this commentary (Goldstein et al, AJKD 2020).
See some completed and ongoing cluster RCTs in dialysis units below
The DIALMAG-CANADA trial will use a cluster randomized design in which all patients within each dialysis unit are randomized as a cluster between high and low dialysate magnesium.
Why do we care about serum magnesium levels in patients with CKD and ESRD on dialysis?
Compared to serum potassium, serum magnesium levels receive much less attention, despite a recognition that hypomagnesemia is a known risk factor for cardiac arrhythmias. Partly this is because studying and modifying magnesium levels is very hard.
Mg is an integral part of ATP, nucleic acids, and a co-factor to hundreds of enzymes. Kidneys play an integral role in maintaining magnesium balance within the body, reabsorbing 75% of filtered Mg and excreting only about 100 mg per day. Extracellular magnesium is estimated to represent only 1% of total body stores. In patients who have ESRD, magnesium concentrations depend on native kidney function, dietary intake, concomitant medications, and dialytic clearance. The interplay between Mg and PTH is very interesting, and plasma Mg has been demonstrated as a significant determinant of serum PTH concentration independently of calcium and phosphorus. Low magnesium levels impair PTH release in response to hypocalcemia but this is unlikely to happen in ESRD patients due to secondary hyperparathyroidism. Low magnesium concentrations are speculated to cause endothelial dysfunction, enhance soft tissue calcification, and promote cardiac arrhythmias. Hypomagnesemia by itself is capable of prolonging QT interval and increasing risk for ventricular Arrhythmias.
Magnesium Counteracts Vascular Calcification. Passive Interference or Active Modulation? Arteriosclerosis, Thrombosis, and Vascular Biology
Moderate hypermagnesemia can also inhibit the secretion of parathyroid hormone (PTH), leading to a reduction in the plasma calcium concentration. In the long term, hypermagnesemia can contribute to osteomalacia, renal osteodystrophy, and adynamic bone disease.
In 2015, a large observational study (Lacson et al, AJKD 2015) reported the association between serum and dialysate magnesium with mortality in hemodialysis patients. They confirmed the association between lower serum magnesium and mortality also previously reported by two observational Japanese studies. With respect to the mechanism of this protective effect of magnesium, it was speculated to be mediated by the effect on vascular calcification, arrhythmias, or some other unknown pathway. More on this on Swap’s AJKD blog post. But remember that magnesium travels together with nutritional status and a whole host of other confounders which cannot be controlled for.
Magnesium and Muscle cramps
A low serum magnesium concentration is linked to more cramps in patients receiving hemodialysis as well as in athletes, older adults, and pregnant women. Patients on hemodialysis have prioritized muscle cramps as 1 of the top 3 physical symptoms that should be targeted for novel therapies. One potential cause of muscle cramping in patients receiving hemodialysis is a low serum magnesium. Hypomagnesemia reduces Na-K ATPase activity thereby reducing the threshold for nerve stimulation and greater chances of involuntary muscle contraction and cramping. Low Mg also impairs Na-Ca exchange transporter leading to greater influx of Ca into muscle cell cytosol and increased excitability. Mg also regulates ROMK channels and Mg depletion causes inhibition of ROMK channels increasing K secretion possibly leading to hypokalemic muscle cramps. Therefore, it is imperative to avoid overlapping risk exposures, such as hypomagnesemia, with hypokalemia. The Dial-Mag investigators wrote an extensive narrative review (Varghese et al, Can J KHD 2020) on higher concentration of dialysate magnesium to reduce the frequency of muscle cramps. This review also covers the potential effect of increasing dialysate magnesium on serum magnesium.
What are the causes of hypomagnesemia in patients receiving hemodialysis?
There are multiple causes for hypomagnesemia in dialysis patients: diet, proton-pump inhibitors, loop diuretics, hypoalbuminemia as well as low dialysate magnesium (see Navarro-Gonzalez et al Semin Dial 2009). The lower the concentration of dialysate magnesium, the more magnesium is removed from the body during hemodialysis. The ideal dialysate magnesium concentration is controversial (see Pun et al, JASN 2017) and the pendulum has shifted from a low Mg dialysate to high Mg dialysates. In the 1970s and 1980s, dialysate magnesium concentrations were more commonly kept at 0.75 mmol/L. As time went on, the concentration was lowered to 0.375 to 0.50 mmol/L, possibly due to beneficial effects seen with a lower dialysate magnesium concentration in relieving uremic pruritus and reducing the risk of osteomalacia.
Why manipulate dialysate magnesium rather than prescribe oral magnesium supplements?
Increased concentration of dialysate magnesium appears to be well tolerated and is associated with a very low risk of symptomatic hypermagnesemia. Raising serum Mg levels with higher Mg dialysate is pragmatic and has many advantages - no additional cost to patients, no pill burden, minimal to no side effects and easy to administer! Oral magnesium can cause diarrhea, which can reduce serum magnesium - making the cure worse than the disease.
What evidence do we have surrounding safety of Magnesium-based interventions in patients receiving hemodialysis?
Symptomatic hypermagnesemia among dialysis patients is uncommon and usually does not occur until Mg is more than 2 mmol/L( 4 meq/L). Increasing the concentration of dialysate magnesium appears to be well tolerated and is associated with a low risk of symptomatic hypermagnesemia. In a small recent trial (Bressendorff et al, cJASN 2018), 59 patients were randomly allocated to higher (1 mmol/l) vs lower (0.5 mmol/L) dialysate magnesium for 4 weeks; the mean serum Mg rose from 0.9 to 1.4 mmol/l.
Black circles indicate dialysateMg of 1.0 mEq/L; white circles indicate dialysate Mg of 2.0 mEq/L
The DialMag-Canada trial
The aim of this trial led by Amit Garg and colleagues is to determine if providing a higher versus lower dialysate magnesium concentration as a centre policy alters outcomes important to patients and their providers.
This is a highly pragmatic cluster randomized, open label, and multicenter trial that will be embedded into routine care in hemodialysis centres in Canada. Centres will be randomized to set the hemodialysate magnesium concentration to be 0.75 mmol/L or ≤ 0.5 mmol/L in the intervention and control groups, respectively. Patients receiving maintenance hemodialysis at these centres will be followed for study outcomes during the trial follow-up period.
How is Dial-Mag Canada a useful example of a cluster RCT?
Modulating dialysate magnesium is a clever strategy: there is little risk of non-adherence (as in the TIME trial discussed above) since most programs and dialysis units do not order a specific dialysate magnesium - it is usually the default in the unit. Who will be bothered if the D-Mg is set at 0.375 or 0.75?
For this same reason, the intervention makes sense as a clustered intervention - it is easier to arrange for a standard dialysate magnesium for an entire unit (especially with central dialysate delivery) rather than randomize individual dialysis patients.
We have seen from the data above that practice patterns vary (e.g. use of different dialysate magnesium concentrations) and that changing dialysate magnesium is a low or minimal risk intervention. Most patients do not know their dialysate magnesium. So forgoing consent (while safeguarding them with all other aspects mentioned above) appears ethical.
This trial plans to enrol 25,000 patients, possibly including most units in Canada and perhaps internationally, with plenty of clusters for a good sample, as discussed above.
Lastly, with the outcomes being cramps (an important PROM) as well as hospitalization and mortality, this becomes an important question worth studying.
Hopefully this trial will improve our understanding of effects of higher dialysate Magnesium- (the often forgotten element) and inform us whether it minimizes mortality as well as muscle cramps which are both very high priorities to improve patient related outcomes.
So in conclusion, cluster randomized trials are quite complex and performing a trial with this design really needs to be justified due to its unique issues with logistics, ethics, biases and methods of analysis. But when performed in the right setting, they can help inform and change group practice and can be quite pragmatic, allowing the research to rapidly roll into clinical practice to benefit patients.
Summary prepared by Manasi Bapat
Nephrologist, CA
NSMC faculty