#NephTrials
June 25, 2025, Space on X
JAMA Netw Open. 2024 Dec 2;7(12):e2449998. doi: 10.1001/jamanetworkopen.2024.49998.
The Global Kidney Patient Trials Network and the CAPTIVATE Platform Clinical Trial Design A Trial Protocol
PMID: 39661388
#NephTrials is an ongoing initiative between #NephJC and the ISN-ACT group. The ISN-ACT (Advancing Clinical Trials) is an International Society of Nephrology (ISN) initiative to encourage existing infrastructures within ISN to improve the global nephrology community’s participation in clinical trial research.
Introduction
Chronic kidney disease (CKD) is quietly, but steadily, climbing the ranks of global mortality, now affecting nearly a billion people and projected to be the fifth leading cause of death by 2040. This isn’t just a statistic- it’s a slow-moving, system-wide crisis, fueled by modern life itself: obesity, hypertension, and type 2 diabetes. (Foreman KJ, et al, Lancet, 2018) For decades, nephrology was a specialty defined more by what we couldn’t do than what we could- hampered by the therapeutic nihilism, excluded from major trials, and underserved by clinical research infrastructure built for simpler questions and shorter timelines.
But that story is changing, and it feels we are living during the Reinaissance. Over the past few years, we’ve seen an unprecedented influx of positive trials- flozins, finerenone, GLP-1 receptor agonists, and more- each showing meaningful, if partial, reductions in kidney and cardiovascular risk. Yet the progress brings new challenges. One drug at a time isn’t enough, as CKD is more than one disease. We now stand at a crossroads where more evidence is not the same as a better strategy. With multiple therapies on the table, the question is no longer “Does it work?” but “What works best, for whom, and in what order?”
Image by Brendon Neuen, adapted from Neuen BL et al, Nephrology Dialysis Transplantation, 2025
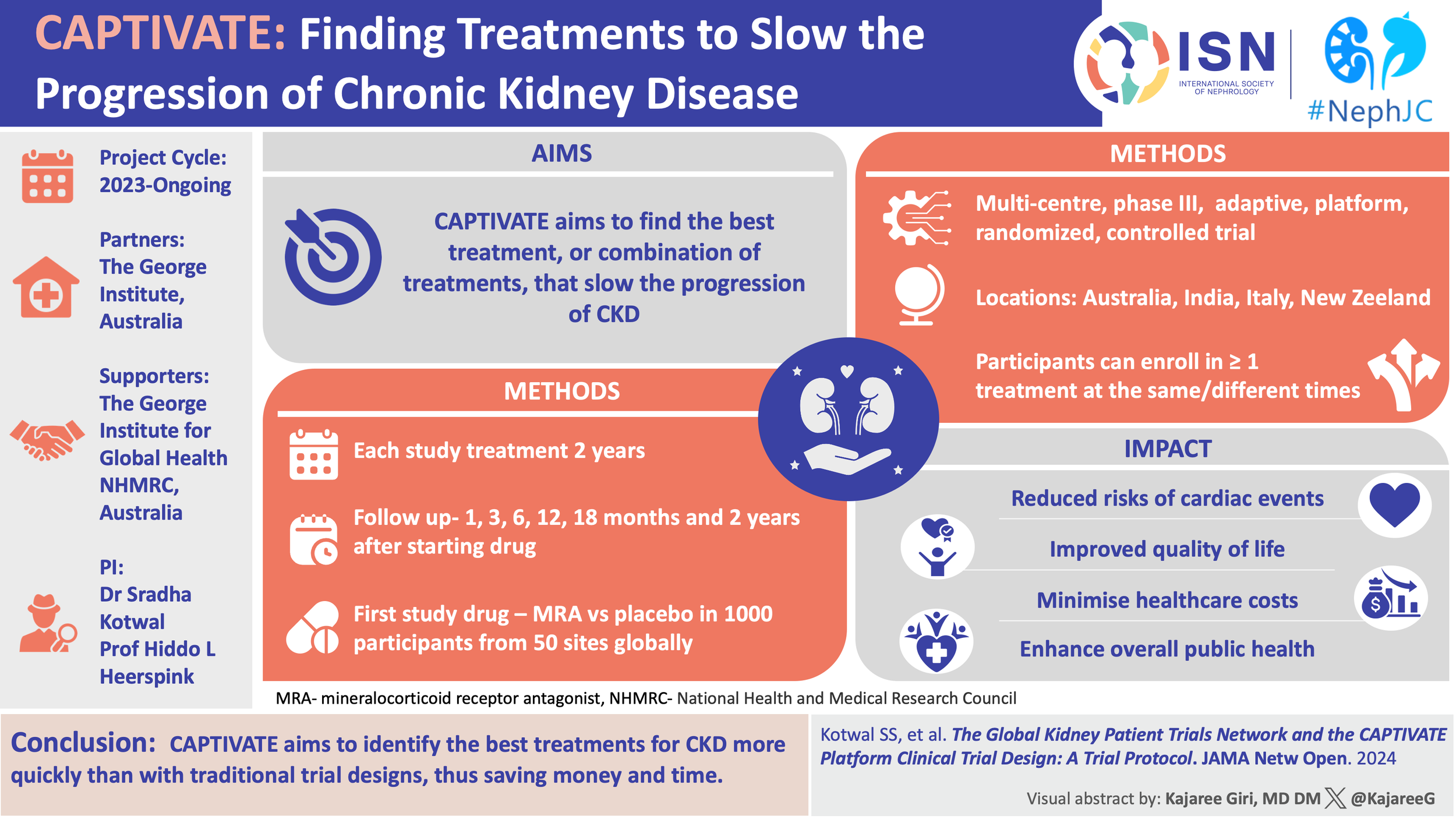
This is where our trial models begin to falter. The rigid structures that brought us proof now struggle to generate wisdom. The sheer number of possible combinations, sequences, and phenotypes overwhelms traditional RCT design. What we need is not just more trials, but better ones. Trials that are nimble, cumulative, and aligned with how we practice. The stage was long prepared for a new kind of trial to CAPTIVATE the nephrology public: an adaptive, registry-based, platform trial purpose-built for CKD. (Kotwal SS, et al, JAMA Netw Open, 2024) Rather than testing one drug in one population for one outcome, it asks a more complex question: what combinations work, in which patients, and when? The trial is not only looking for tidy answers but rather for better questions.
Can a global CKD registry improve both trial efficiency and external validity?
Yes- and CAPTIVATE is designed to prove it. At the heart of this trial is the Global Kidney Patients Trial Network (GKPTN), a prospective registry, started in May 2020, of over 4300 patients with non-dialysis CKD across 8 countries, from nephrology and endocrinology practices. The registry serves a dual purpose: first, as a feeder cohort, it allows trial sites to pre-identify eligible participants using structured clinical data already captured in real-world care. Second, it functions as a mirror cohort, tracking long-term outcomes in non-randomized patients, which helps contextualize trial findings and supports external validity.
Inclusion into GKPTN requires a documented diagnosis of primary kidney disease (e.g. diabetic kidney disease, IgA nephropathy, FSGS, membranous nephropathy), eGFR >15mL/min/1.73m2, and regular nephrology follow-up (every 6-12 months). Children ≥2 years may be enrolled, but CAPTIVATE only includes adults. Exclusion criteria include current dialysis, expected start of renal replacement therapy within 6 months, or short life expectancy.
eTable 1. Inclusion and exclusion criteria, from Kotwal SS, et al, JAMA Netw Open, 2024
The GKPTN collects eGFR, albuminuria, treatments, comorbidities, and demographics using harmonized case report forms with all data pooled from medical records during routine clinical visits. Linear mixed effects models are used to estimate individual and group-level eGFR trajectories. Because these data are drawn from usual care, they anchor the trial in the real world. This dual function, operational and analytic, means that CAPTIVATE starts from an already mapped clinical landscape. That matters, especially when trials increasingly struggle with recruitment and generalizability.
What makes CAPTIVATE a platform trial, and how is it structured?
CAPTIVATE is not a single trial; it’s a multicenter, perpetual trial platform with a core protocol and modular domain-specific appendices (DSAs, though not donor specific antibodies!). The core protocol defines the overarching eligibility, primary and secondary endpoints, statistical framework, visit schedules, and ethics governance. Interventions were added via DSAs, each defining a therapeutic “domain”, with its own specific inclusion criteria, sample size, randomization scheme, stopping rules, and any additional endpoints. Participants can be enrolled into multiple concurrent DSAs if eligible, as well as sequentially into multiple domains. This allows CAPTIVATE to test more than one mechanistic hypothesis per participant. Sites can activate one or more domains depending on capacity and regulatory approvals, giving flexibility while maintaining consistency through the shared core protocol.
Figure 1. The Chronic Kidney Disease Adaptive Platform Trial Investigating Various Agents for Therapeutic Effect (CAPTIVATE) Study Design from Kotwal SS, et al, JAMA Netw Open, 2024
Previous #NephTrials of the platform trial design and master protocols are linked below:
What endpoints define success, and how are they measured?
CAPTIVATE uses chronic slope from week 4 to week 104 as its primary endpoint. This slope is estimated from local creatinine values using the 2021 CKD-EPI equation and modeled via Bayesian mixed models. Excluding the first four weeks helps isolate true disease-modifying effects from early hemodynamic changes.
Courtesy of Brendon Neuen
The key secondary endpoints, standardized across DSAs unless otherwise specified, include: change in UACR at 24 weeks, change in eGFR from randomization to end of washout (week 108), composite of ≥40% eGFR decline or kidney failure by week 108 (eGFR <15 or start of chronic renal replacement therapy), time to ≥40% eGFR decline or kidney failure, all cause mortality at week 108, time to cardiovascular event (CV death, hospitalized HF, MI, stroke), adverse events, changes in quality of life (using QDIS-CKD at 6 months intervals).
eTable 2. Primary and secondary outcomes, from Kotwal SS, et al, JAMA Netw Open, 2024
Additional exploratory outcomes include healthcare resource use, cost-effectiveness, and composite win ratios incorporating mortality, kidney failure, and eGFR outcomes.
How does the adaptive design work? When do we stop for futility or success?
CAPTIVATE uses Bayesian interim analyses, typically conducted every 6 months. Each DSA is analyzed independently. There are separate thresholds for early futility and success.
Futility (early stop)
UACR: if the probability of a ≥ 25% reduction at 6 months is low.
eGFR slope: if estimated benefit is <0.8 mL/min/1.73 m2/year by 12 months.
Success (early stop):
Posterior probability >0.985 that intervention improves eGFR slope >2.0 mL/min/1.73 m2 per year
Requires at least 100 participants per arm with complete 108-week follow-up.
Figures 2 and 3 show how CAPTIVATE’s adaptation design uses accumulating data to guide trial decisions over time. In Figure 2, the first interim analysis at 18 months shows that over 100 participants per arm have completed 6 months of follow-up. The intervention achieves 31% UACR reduction with high probability of clinical benefit, allowing the trial to continue. However, the eGFR slope estimate, while numerically favorable, has wide confidence intervals and insufficient follow-up to assess success or futility. At 24 months (fig 2), second interim, enrollment is complete, UACR benefits persist, and the eGFR slope difference increases to 1.38 mL//min/1.73 m2/year. Yet again, while statistically promising, not enough participants have completed 108 weeks follow-up to meet the success threshold. The domain continues.
Figure 2. Interim analyses for 1 domain within the CAPTIVATE platform after 18 and 24 months, from Kotwal SS, et al, JAMA Netw Open, 2024
In Figure 3, at 30 months, the eGFR slope shows strong benefit with 99% posterior probability of superiority, but few patients have reached the full follow-up duration. By 36 months, the final requirement is met: UACR benefit remains robust, > 108 patients per arm completed 108 weeks, and the posterior probability of eGFR benefit exceeds 0.985. The intervention met the prespecified success criteria, and it was closed.
Figure 3. Interim analyses for 1 domain within CAPTIVATE platform after 30 and 36 months, from Kotwal SS, et al, JAMA Netw Open, 2024
The design avoids premature declarations while conserving resources and patient exposure to ineffective therapies.
Who keeps the platform on course?
In a complex, modular trial like CAPTIVATE, coordination is a requirement. Therapeutic conduct spans multiple countries, therapeutic domains, and stakeholder groups. To manage this, CAPTIVATE employs a layered governance structure designed to ensure scientific rigor, participant safety, and operational feasibility across the entire platform.
Figure 2, Supplement 2. Trial ‘organigram’,from Kotwal SS, et al, JAMA Netw Open, 2024
The Platform Oversight Committee (POC) is the trial’s central authority. It includes the platform PIs, trial statisticians, regional leads, operational staff, and consumer representatives, ensuring a mix of expertise in trial conduct, CKD therapeutics, and patient engagement. The POC is responsible for: strategic direction of the platform, reviewing and approving new interventions, regions, and protocol changes, coordinating with data safety monitoring board (DSMB), and overseeing data sharing, interpretation, and publication.
Each DSA is governed by its own DSA steering committee, which oversees design, feasibility, recruitment, and DSA-specific updates.
The statistics and methodology committee includes statisticians and methodologists with expertise in adaptive platform design. They advise on Bayesian modeling, response-adaptive features, and trial simulations.
A single, independent Data Safety Monitoring Board oversees participant safety and the scientific integrity of the platform. It reviews unblinded safety and operational data and makes recommendations to the POC, but the POC retains the final authority on trial continuation, modification, or stopping.
Which interventions are being tested, and how are domains built?
The first domain launched in CAPTIVATE is evaluating finerenone vs placebo, layered on top of standard-of-care (RAS inhibition + Flozin). This tests whether adding a nonsteroidal MRA yields incremental kidney protection. A few hundred patients in the finerenone trials and the FIDELITY analysis were on flozins, which was analyzed as a subgroup. The benefit in the subgroup was consistent as the primary analysis, but it was underpowered on its own, which makes this a promising first domain to test.
Each new DSA must meet a set of predefined criteria:
strong mechanistic rationale
operational feasibility (supply chain, logistics, safety monitoring)
endorsement by the platform oversight committee and the domain steering group
Future DSAs may explore:
head-to-head comparisons
combination therapies
biomarker-guided assignment or rescue randomization for non-responders.
Importantly, DSAs retain enough autonomy to tailor statistical methods or endpoints while staying within the platform.
Is the trial adequately powered to influence clinical practice?
Yes, each domain is powered for decision-making, not just detection. Each arm targets 500 participants (N=1000 per domain). This provides approximately. 90% power to detect a 2.6 mL/min/1.73 m2 difference in eGFR slope over 2 years- equivalent to ~1.3/mL/min/year.
The effect size was selected based on meta-analyses linking slope change to hard outcomes: a ≥ 2 mL/min/1.73 m2 improvement over 2 years correlates with a 20-30% reduction in ESRD risk, a threshold considered to be meaningful. (Heerspink HSL, et al, Lancet Diabetes Endocrinol, 2019)
Since CAPTIVATE used Bayesian modeling, interim analyses can occur without inflating type I error. Instead of dichotomous p-values, it asks how confident we are that this intervention works or doesn’t.
Can this trial design work in real-world CKD clinics?
That’s the goal, and CAPTIVATE has made smart design choices to increase feasibility:
Study visits align with routine care, reducing added burden
Local labs are used
The core protocol’s inclusion is broad: eGFR ≥ 25, any CKD etiology, no age cap (as long as ≥18), real-life comorbidities
Exclusions are minimal and pragmatic: current dialysis, planned transplant, or life expectancy <6 months. Sites choose which DSAs to activate based on interest and resources.
The result is a scalable platform, real-world aligned, and capable of generating pragmatic evidence that nephrologists can apply in practice.
RESULTS
What have we learned so far?
As far as the GKPTN is concerned, a total of 4334 participants were recruited from 119 clinical practice sites in 8 countries (US, Canada, Australia, Italy, Spain, Argentina, China, Japan). Majority patients had a white ethnicity (52.3%), followed by Hispano/Latino (20%), Black/African American (14.4%), Asian (12.3%) and others (1%). Mean age at participant enrolment was 64.5 years, with ~60% being female. Most (43.4%) had diabetic kidney disease, followed by hypertension (30.7%) and glomerulonephritis (23.7%).
The baseline data revealed important gaps between evidence and practice. Despite a cohort marked by high-risk clinical features, the uptake of guideline-recommended was remarkably low:
72.6% of participants received RASi
13.4% received an SGLT2 inhibitor
2% received MRAs
GLP-1 receptor agonists are too early to even make it to table 1
Mean eGFR was 52.9 ml/min/1.73m2. Mean UACR was 89 mg/g. ~70% had eGFR <60 ml/min/1.73m2. 23.5% of participants had UACR >300 mg/g, consistent with high renal and cardiovascular risk. Over a median 0.89 year of follow-up, mean annual change in eGFR was -1.67 ml/min/1.73m2, not varying in the age-specific and sex-specific subgroups, however showing a steeper decline in those with >60 ml/min/1.73m2 and more severe albuminuria subgroups.
Table 1. fromKotwal SS, et al, JAMA Netw Open, 2024
Table 2 shows modeling eGFR decline from the GKPTN registry also adds on:
In the overall cohort, the mean annual eGFR decline was −1.67 mL/min/1.73m², reinforcing a high-risk trajectory despite low medication use.
Decline rates were deeper in those with higher baseline eGFR (−2.29 vs. −1.16 mL/min/1.73m² in patients above vs. below 60 mL/min/1.73m²).
As expected, greater albuminuria was associated with faster decline.
Table 2. fromKotwal SS, et al, JAMA Netw Open, 2024
With respect to CAPTIVATE, about 132 participants have been enrolled so far. The first domain, finerenone vs. placebo, added to standard of care (RAS inhibition + SGLT2i), was launched in late 2024, marking the operationalization of the platform. Other DSAs are currently in development, focusing on comparisons and adaptive assignment strategies.
Simulations of the adaptive framework, particularly interim analyses, suggest that:
UACR-based futility rules can lead to early termination of non-effective arms, reducing patient exposure and conserving trial resources.
Posterior probability modeling provides a more flexible and decision-relevant framework than rigid frequentist thresholds.
Are Interim Analyses Adapting Over Time?
As outlined in the Methods, CAPTIVATE’s Bayesian interim analyses are designed to evolve with the data. Across the second to fourth analyses within the first domain, we observe a glimpse into the potential of adaptive trials to guide decisions in real time. Emerging a continuous feedback loop:
Consistent and sustained reductions in UACR.
Progressive refinement of eGFR slope estimation.
The domain remained active until more than 100 participants per arm completed 108 weeks of follow-up, at which point the posterior probability of eGFR benefit exceeded 0.985, meeting the predefined threshold for success.
This trajectory avoids premature conclusions, evolving with the data and offering not just answers, but decision-making in CKD progress.
How will CAPTIVATE address combination therapy and interaction effects?
The multifactorial design enables simultaneous assignment to multiple domains.
Analyses assume independence unless interaction is biologically or statistically
Future domains might explore:
GLP-1 RA
ERA (endothelin receptor antagonist)
Join us tonight on the X-space to explore this captivating trial design with Dr Kotwal and the ISN-NephJC team!
*If you couldn’t be there, you can listen to the CATIVATE-ing discussion via 👇link 👇
Summary prepared by
Cristina Popa, Sayali Thakare, and Milagros Flores
Reviewed by Swapnil Hiremath and Sradha Kotwal