#NephJC Chat
Tuesday February 26 9 pm Eastern
Wednesday February 27 9 pm IST
Wednesday February 27 8 pm GMT, 12 noon Pacific
N Engl J Med. 2019 Jan 31;380(5):447-458. doi: 10.1056/NEJMoa1810742. Epub 2018 Oct 26.
Intravenous Iron in Patients Undergoing Maintenance Hemodialysis.
Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray JJV, Murray H, Tomson CRV, Wheeler DC, Winearls CG, Ford I; PIVOTAL Investigators and Committees.
PMID: 30365356 Full Text at NEJM
Introduction
Anyone dealing with CKD and dialysis patients knows that anemia is one of the most common complications and is associated with significant morbidity and mortality. Treatment options include iron supplementation (oral or IV) and erythropoeitin stimulating agents (ESAs). The enthusiasm for prescribing ESAs has been somewhat dampened by an association with cardiovascular, thrombotic, and neoplastic complications. NephJC covered the debacle of the effect of ESAs on quality of life here. There truly has been a medical reversal in the use of ESAs and anemia targets in the last decade. One explanation for the counter intuitive finding of harm from high hemoglobin was the possibility that it wasn’t the ESA, but rather the higher iron usage in the normal hematocrit arm, driving the higher cardiovascular events. (Seriously, read the abstract and/or the discussion from the Normal Hematrocrit Study).
Iron supplementation is used to treat anemia without increasing the use of ESA or the need for blood transfusions. NephJC previously covered the oral vs IV iron debate, from the REVOKE trial. However, there is no consensus on what is considered an adequate dosing of iron. Moreover, the dose of IV iron has been restricted by fears of iron toxicity, increased risk of infection, oxidative stress and atherothrombosis. Thus the KDIGO guidelines do not set an upper limit for iron therapy and instead focus on a lower limit at which iron therapy may be initiated [Transferrin Saturation(TSAT) ≤ 30% and Ferritin ≤500mg/ml ]. This has lead to these lower limits being used, perhaps somewhat erroneously as upper limits.
“The ferritin floor has been mistaken as the ceiling.”
Ferritin may also be elevated in response to inflammation so a high ferritin is not synonymous with iron adequacy. You can read the excellent KDIGO report on the controversies conference on iron management in CKD here. The DRIVE trial by Coyne et al randomized patients with a high ferritin and low TSAT to either no iron or IV iron. The trial showed that haemoglobin increased more and at a faster rate with IV iron and that the rise in haemoglobin was unrelated to the ferritin level. Beyond the DRIVE study, there is little high quality, RCT data looking at hard clinical outcomes with the use of IV iron in dialysis patients. This systematic review of the available evidence concluded that higher-dose IV iron does not seem to be associated with higher risk of mortality, infection, cardiovascular events, or hospitalizations in adult patients on dialysis. However, as the authors noted, the trials had a small number of events - and the data from the observational studies have incongruent results. All of this sets the stage quite nicely for the PIVOTAL trial.
The Study
Study Design
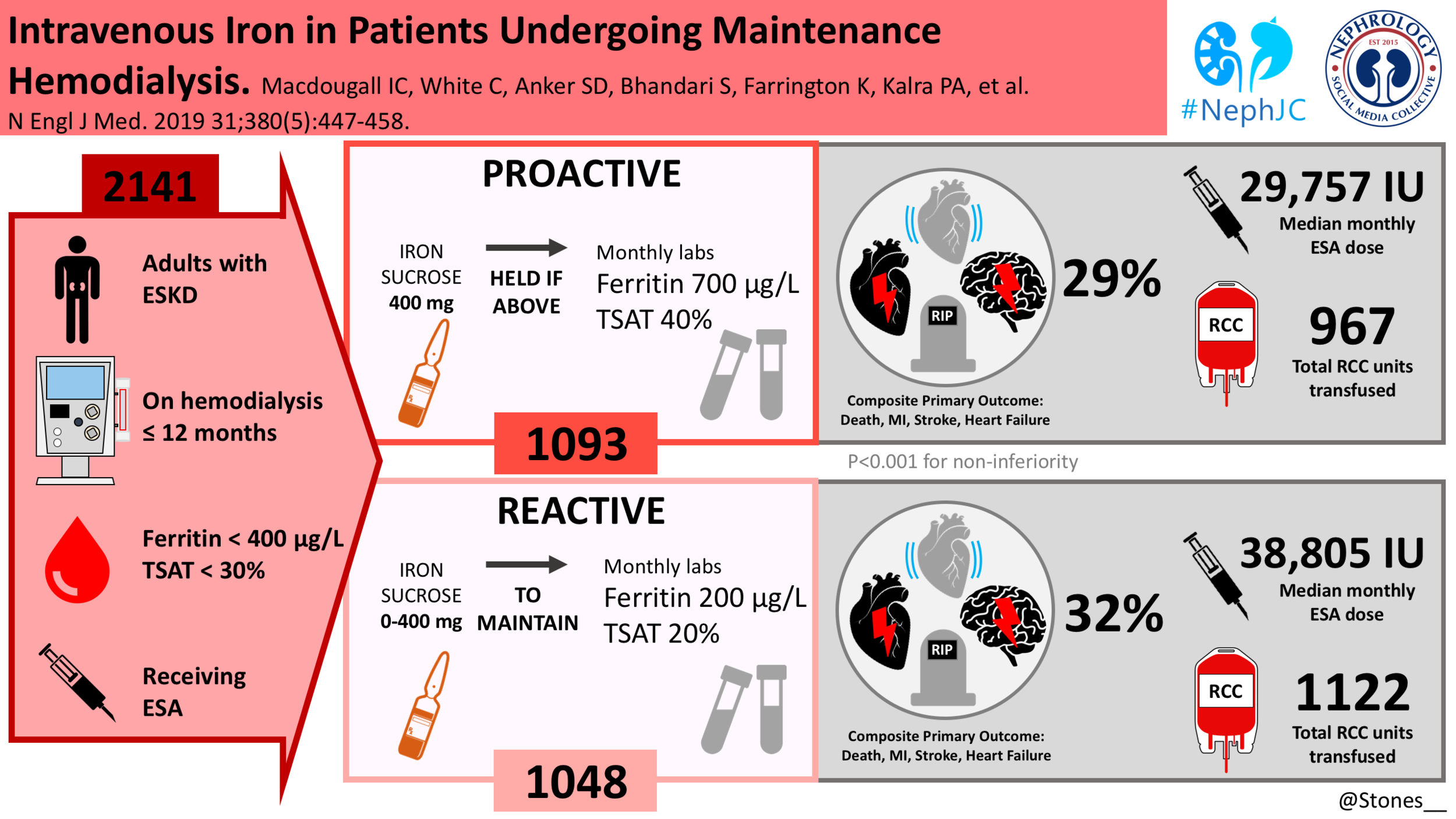
This was a prospective, randomized, open-label, blinded end-point, controlled trial at 50 sites in the United Kingdom. An independent data and safety monitoring committee performed regular safety surveillance. Patients were randomly assigned to high-dose intravenous iron administered proactively or low-dose intravenous iron administered reactively. Randomization was stratified according to vascular access (dialysis catheter vs. arteriovenous fistula or graft), diagnosis of diabetes (yes vs. no), and duration of hemodialysis treatment (<5 months vs. ≥5 months).
Inclusion criteria
Age ≥18yrs
Initiated on maintenance hemodialysis within 12 months of the screening visit
TSAT ≤30%
Ferritin level ≤400mg/ml who
Receiving any ESA. Any iron therapy that had been prescribed previously was discontinued at the screening visit.
Exclusion criteria
Life expectancy < 12 months
Living-donor transplant scheduled within the next 12 months
CRP > 50 mg/L
Active infection
Active malignancy
Chronic liver disease
Advanced heart failure (NYHA IV)
Pregnancy or breast feeding
History of acquired iron overload
Previous severe hypersensitivity
Intervention
The proactive regimen: In the high-dose group, 400 mg of IV iron sucrose per month, was prescribed to the patients, with safety cutoff limits (ferritin concentration of 700 μg/liter (mg/ml) or a TSAT of 40%) above which further IV iron administration was withheld pending repeat testing performed monthly for the duration of the study.
The reactive regimen: Patients in the low-dose group received a monthly dose of 0 mg to 400 mg of iron sucrose as required to maintain a minimum target ferritin concentration of 200 μg/liter (mg/ml) and a TSAT of 20%.
Iron therapy was temporarily withheld if the trial team identified an active infection.
Endpoints
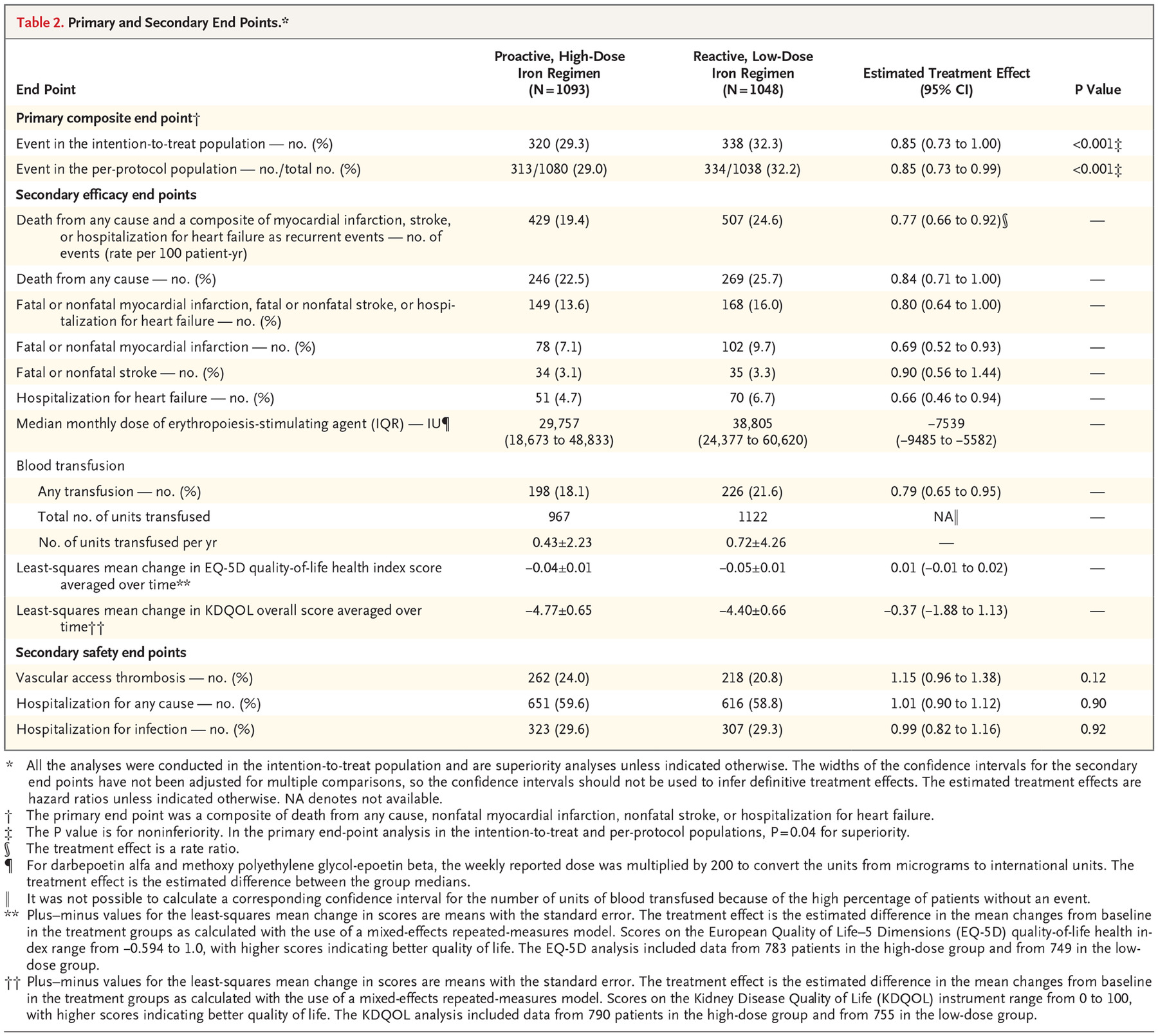
Primary end-point: Time to all-cause death or a composite of non-fatal cardiovascular events (myocardial infarction, stroke, and hospitalization for heart failure).
Secondary end-points:
Incidence of all-cause death and a composite of non-fatal myocardial infarction, stroke, and hospitalisation for heart failure as recurrent events.
Time to (and incidence of) all-cause death, composite cardiovascular event, myocardial infarction, stroke and hospitalisation for heart failure
ESA dose requirements
Transfusion requirements
EQ-5D QOL and KDQOL
Safety end-points:
Vascular access thrombosis
All-cause hospitalisation
Hospitalisation for infection
Infection episodes
Analytic plan and sample size
This was designed as a non-inferiority study. The authors assumed a 3-year event rate of 40% in the low-dose group and a 10% loss to follow-up (including loss to follow-up due to kidney transplantation). For this with 80% power, they estimated that a sample of 2080 patients who had 631 primary end-point events would suffice to assess the noninferiority of high-dose iron to low-dose iron, with a noninferiority limit for the hazard ratio of 1.25. If noninferiority was established, a two-sided superiority test (Wald statistic) was carried out with no penalty regarding the P value.
Funding
The trial was funded by Kidney Research UK, which was supported by an unrestricted grant from Vifor Fresenius Medical Care Renal Pharma (which also provided iron sucrose for the trial, free of charge). Vifor Fresenius Medical Care Renal Pharma had no input into the trial design or the data collection or analysis. However, the company was kept abreast of the progress of the trial by regular study reports and newsletters. No confidentiality agreements regarding the data were in place.
Results
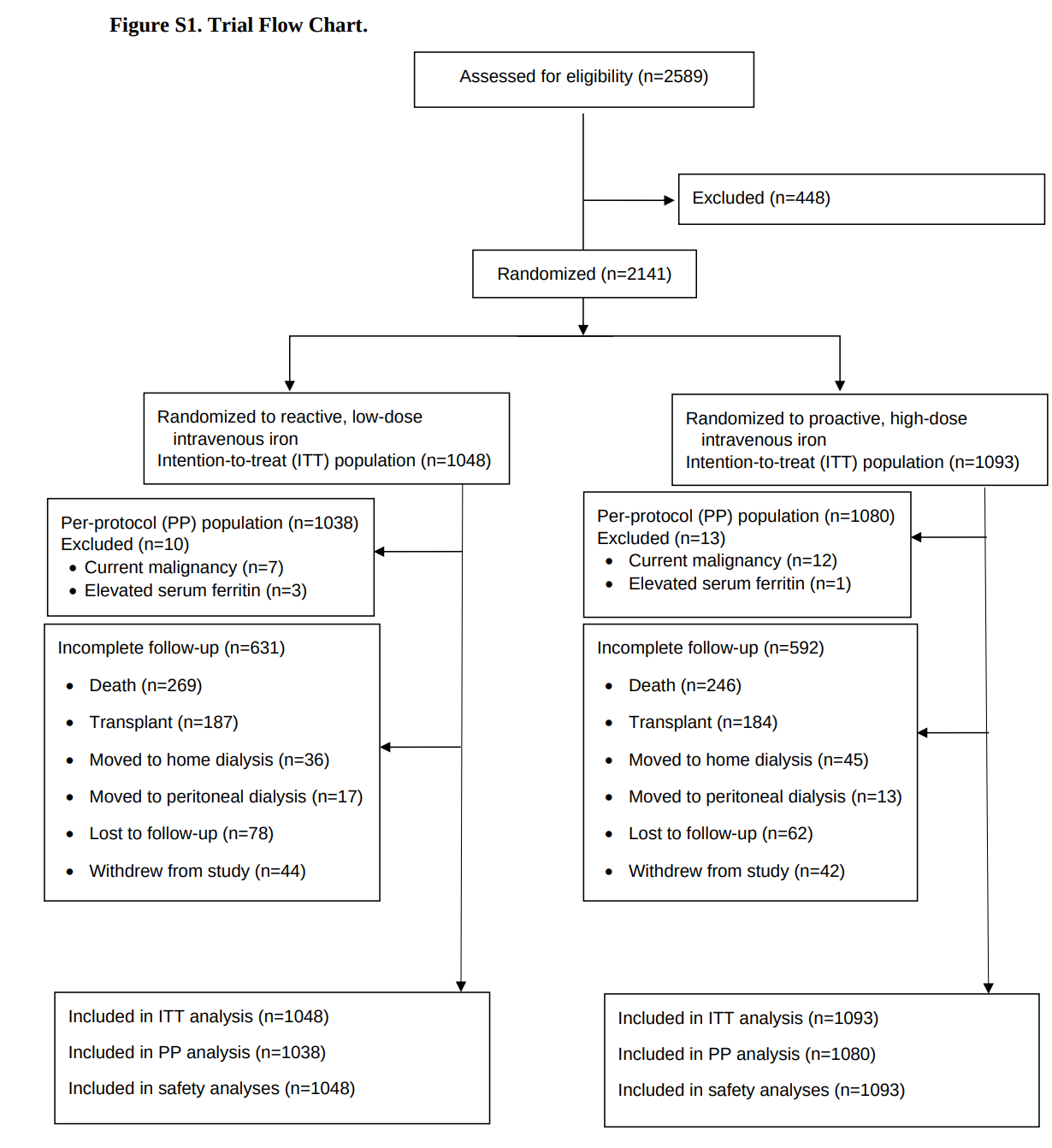
The trial was conducted from November 2013 to June 2018. 2141 patients were randomly assigned to either one of the treatment groups (1093 patients to the high-dose group and 1048 to the low-dose group) and constituted the intention-to-treat population.
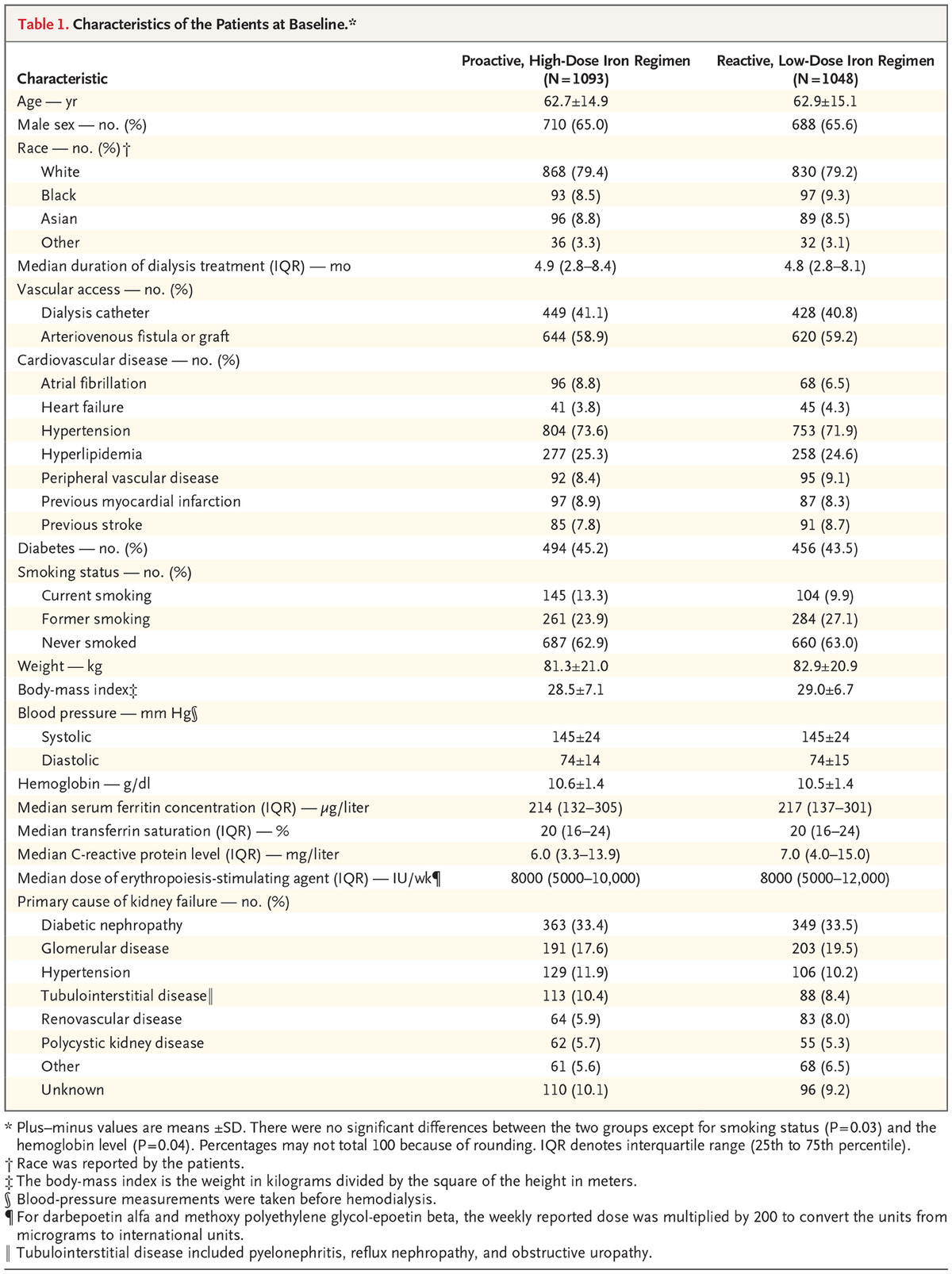
Table 1 shows baseline characteristics, with no surprises, except for a slightly higher haemoglobin in the high dose IV iron group and smoking status. The median duration of follow up was 2.1 years and maximum follow-up was 4.4 years.
The high-dose IV iron group expectedly received higher doses of iron, a median of 2000mg more than the control group at 12 months. The median monthly dose was also higher 264 mg in the high dose group vs 145mg in the low-dose group.
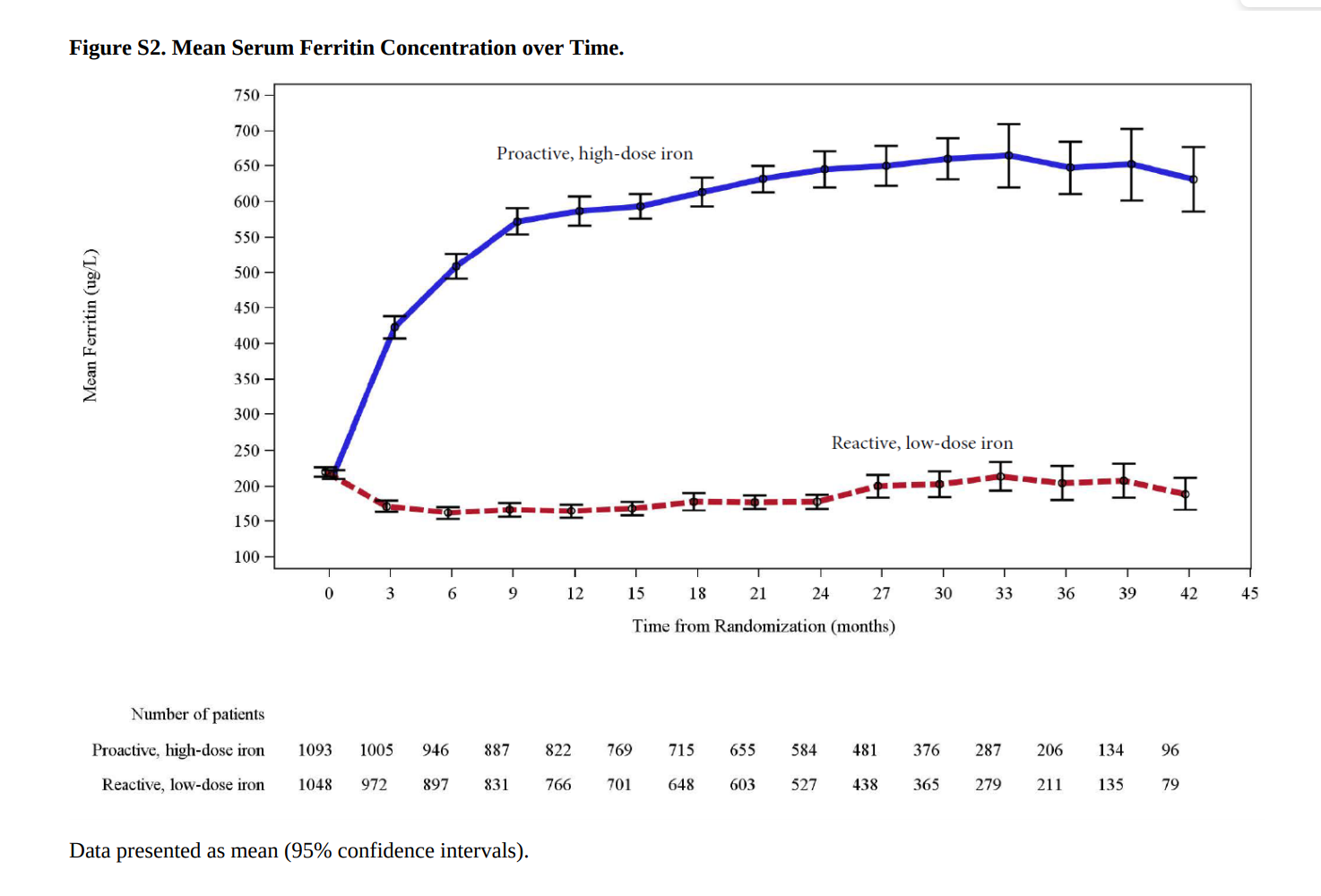
The ferritin and transferrin saturation both had a much steeper rise from baseline in the high dose group.
Tellingly the cumulative doses of ESAs were significantly lower at all post baseline time points in the high dose group. Median of 29,757 IU per month in the high dose regimen vs 38,805 IU per month in the low dose regimen.
Given all this, what would you expect to happen with the hemoglobin? Remember that the hemoglobn targets were similar: so more iron resulted in a faster rise in hemoglobin, but the reactive group caught up due to the effect of the higher ESA doses, as seen below.
Primary end point:
A primary end-point event occurred in 320 patients (29.3%) in the high-dose group, as compared with 338 (32.3%) in the low-dose group (hazard ratio, 0.85; 95% CI, 0.73 to 1.00; P<0.001 for noninferiority; P=0.04 for superiority). This effect was persistent across all pre-specified subgroups and after censoring the data for patients who discontinued iron therapy.
Secondary end-points:
Death rates between the 2 groups were similar: 246 deaths in the high-dose group vs 269 in the low dose group (hazard ratio, 0.84; 95% CI, 0.71 to 1.00)
The rate of the composite of fatal or nonfatal myocardial infarction, fatal or nonfatal stroke, or hospitalization for heart failure was lower in the high-dose group than in the low-dose group (hazard ratio, 0.80; 95% CI, 0.64 to 1.00).
Patients in the high-dose group received less blood transfusions than those in the low-dose group (hazard ratio, 0.79; 95% CI, 0.65 to 0.95).
There were no significant between-group differences with regard to changes from baseline in either the EQ-5D quality-of-life health index or the Kidney Disease Quality of Life overall score.
Safety end points:
Vascular access thrombosis was slightly higher in the high dose group 24.0% vs 20.8%
Hospitalization for any cause and for infection were similar in the two groups.
The rate of all episodes of infection was lower in the high-dose group was 63.3 events per 100 patient-years vs 69.4 events per 100 patient-years in the low-dose group (rate ratio, 0.91; 95% CI, 0.79 to 1.05).
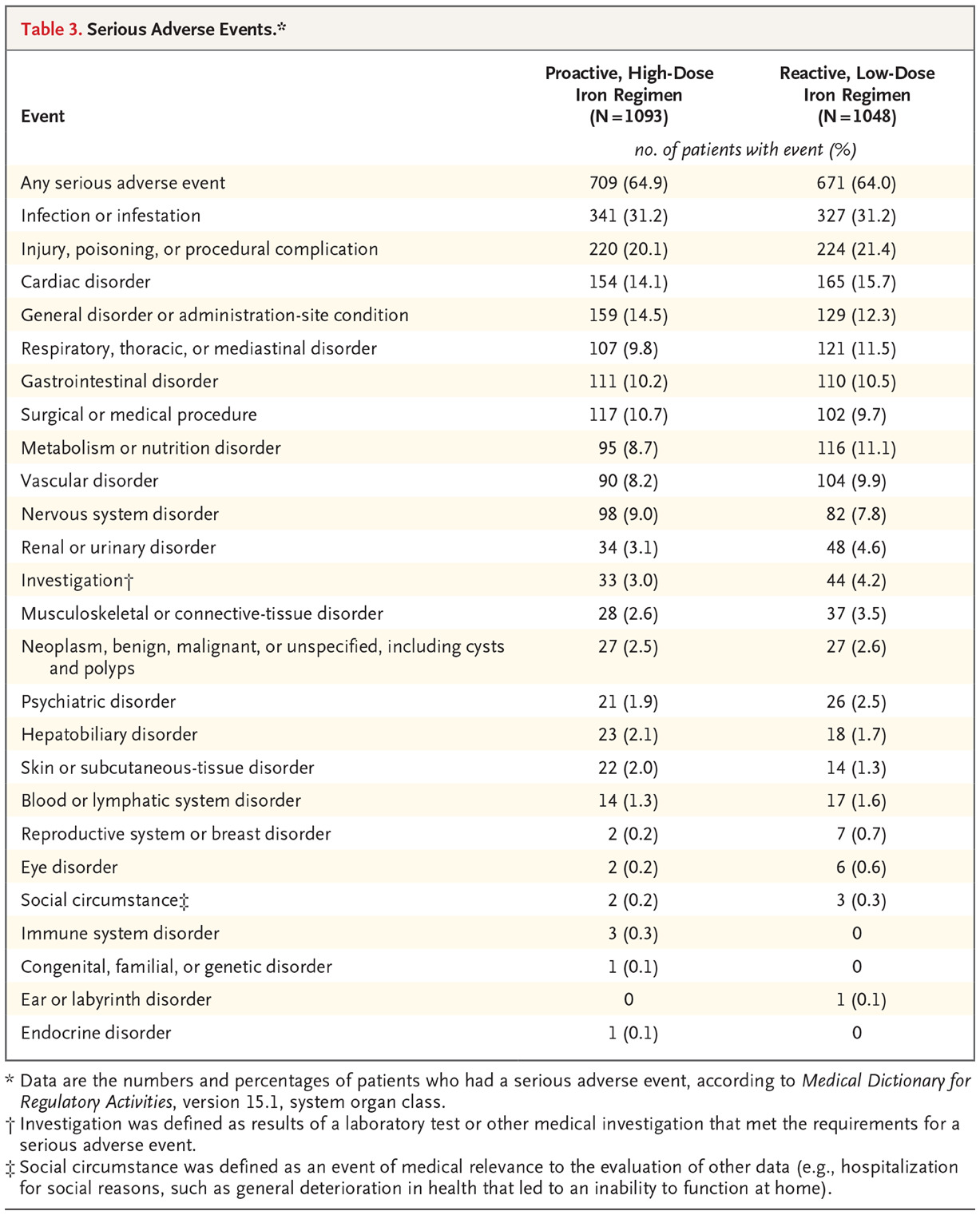
These results are different than the ones initially presented at KidneyWk and published in NEJM. Though the proactive iron was reported to be non-inferior, it was not reported as being superior (p = 0.11 for the primary outcome). However, the analytic team mistakenly used the results for nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure based on the investigators’ judgments rather than on adjudicated events. That’s why the results presented above report that the proactive iron was not just non-inferior, but also superior for the primary, and some secondary outcome. These events were adjudicated by a blinded Endpoint Adjudication Committee, so the correction seems quite above board.
Discussion
The PIVOTAL study group concluded that the use of a high-dose intravenous iron regimen administered proactively was non-inferior to the use of a low-dose intravenous iron regimen administered reactively and was not associated with higher risks of death, major adverse cardiovascular events, or infection.
This validates the findings of the DRIVE study. Dr Daniel Coyne (lead author of the DRIVE study) (smugly, or with a sigh of relief!) summarised the impact of the study here, as well as in a follow up review.
Why do we see the results we do? Is more iron protective - or perhaps more ESA is harmful? From 1998, when more iron was blamed for the higher event rate in the Normal Hematocrit Trial, we have now pivoted 180 degrees to more iron being beneficial, and more ESA being harmful. The medical reversal is complete.
Limitations
The trial was open label - and indeed it is hard to perform a blinded trial requiring doses to be adjusted based on routine lab values. The outcomes were adjudicated by a blinded committee, which is what matters.
Secondly, the trial was conducted in hemodialysis patients, limiting its generalizability to patients with CKD not on dialysis, and to peritoneal dialysis.
Lastly, the trial had a relatively short follow up of a median of 2.1 years - but the large number of events should reassure us about this aspect.
Conclusions
Should we accept these results and become proactive? It does certainly suggest, that holding to a low ferritin threshold is not being cautious, but being cavalier from a cardiovascular point of view.
How should this change our anemia strategy? Daniel Coyne, in his review of the findings suggests that we should stop considering any upper limit of ferritin. Yes, patients with active infections should not get iron, but avoiding iron once the TSATs rise above 40-50% would also obviate the hemochromatosis risk. Otherwise, keep on pumping iron.
The findings of the PIVOTAL study should settle some of the debate surrounding the safety and efficacy of IV iron usage in hemodialysis patients and hopefully lead to a change in the guidelines and clinical practice.
Summary by Sanjeev Nair, Nephrologist, Chennai