#NephJC Chat
Tuesday August 6, 2019 at 9 pm EDT
Wednesday August 7, 2019 at 9 pm IST
Wednesday August 7, 2019 at 9 pm BST, 1 pm Pacific
JAMA. 2019 Jun 6. Doi: 10.1001/jama.2019.5774. [Epub ahead of print]
Reconsidering the Consequences of Using Race to Estimate Kidney Function.
PMID: 31169890 Full Text at JAMA (limited time free access)
Additional Reading
Original CrCl/GFR equation papers
Cockroft and Gault, Nephron, 1976
MDRD Equation, Annals of IM, 1999
CKD-EPI, Annals of IM, 2009
CKD-EPI 2011 validation, KI
Blog post on PB Fluids on the controversy
Discussion
Do eGFR equations insidiously lead to discrimination on the basis of race?
At first, this seems implausible because we accept that black people have a higher serum creatinine and that this is some way related to somatic muscle mass and that our eGFR equations (which only provide an estimate of true GFR) must account for this. But what if this simply is not true? What if our broad stroke use of eGFR equations in ethnically diverse populations who do not fit specific ‘traditional’ racial categories is affecting health outcomes and access to healthcare?
At present, it is standard clinical practice for labs to provide an eGFR (e for estimated or calculated using either the MDRD or CKD-EPI equation). eGFRs are used to inform numerous clinical decisions:
When to refer to nephrology
Dosing of antibiotics and other medications
When is it safe to use iodinated and gadolinium-based contrast agents
When can patients be listed for a kidney transplant
The timing of dialysis planning and initiation
Inclusion in clinical trials
The commonly encountered eGFR calculations are based on a single biochemical measurement, serum creatinine, and three demographic variables: race, sex, and age.
How - and why - did race become part of the formula?
Older methods for estimating kidney function did not include race as a variable. The Cockcroft-Gault formula (for creatinine clearance) was based on age, sex, and body weight. In the 1990s, investigators noted a limitation for Cockcroft-Gault in that it did not perform well as an estimate of creatinine clearance in black patients. There were other problems with Cockroft-Gault: one needs a weight, for one - is it lean weight, or ideal body weight? What is someone has ascites - or paraplegia, or an amputation? It was clear, therefore, that a more refined approach to eGFR calculation was needed and this is where MDRD and CKD-EPI came in. MDRD was a spinoff from the original MDRD trial - which was negative in showing any effect of low protein intake or intensive BP lowering, but left a lasting legacy stemming from the measurement of nuclear GFR in all participants.
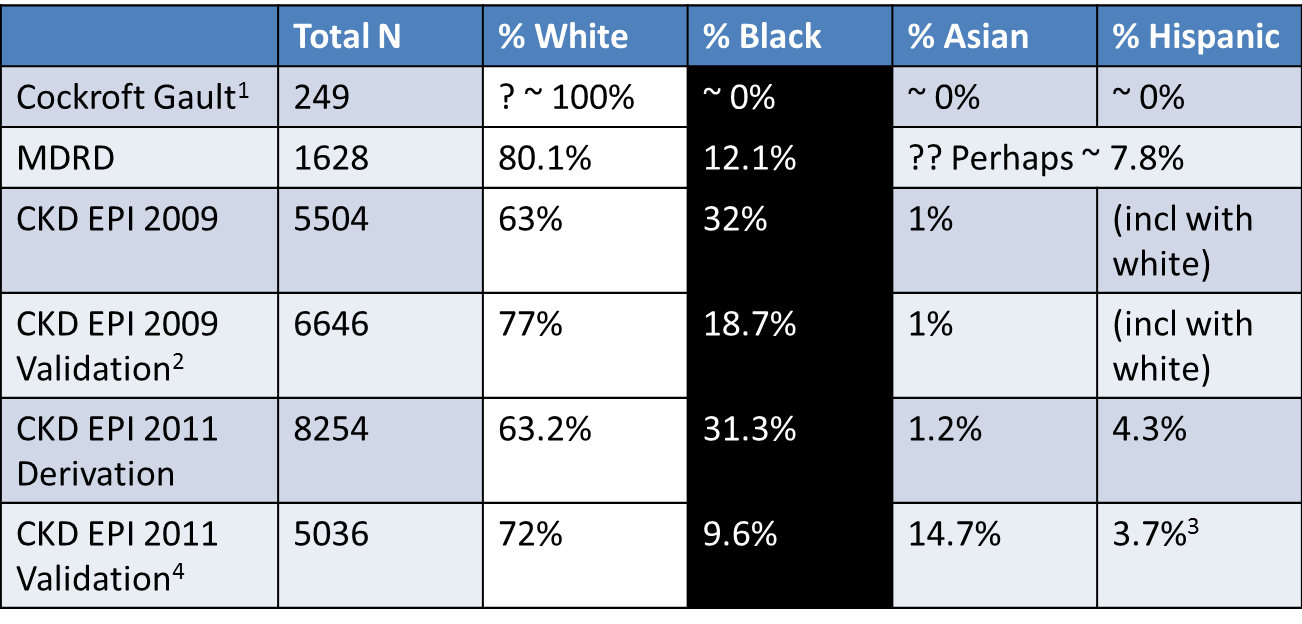
Proportion of participants by race/ethnicity in the GFR estimating equations datasets
1 not reported, assumed to be mostly white based on location of study. Incidentally, also > 96% men, but reported using 15% lower factor for women
2 Includes and internal and external validation datasets combined here
3 includes North American Indians
4 Includes US/Europe and Non-US/Europe (China, Japan and South Africa) datasets combined here
It is frequently assumed that the higher serum creatinine observed in black persons is secondary to ‘greater muscle mass’. Indeed, a search of PubMed on this particular issue will yield a collection of anthropometric studies to support this. What is less certain is whether more skeletal muscle in black persons truly accounts for higher serum creatinine across the spectrum of chronic kidney disease. In a 2008 study, Hsu et al took almost 3000 hemodialysis patients (~50% of the population were black) and sought to determine if the higher serum creatinine observed in black patients was secondary to body composition (assessed by bioelectrical impedance). Interestingly, they found no such association (after controlling for age, nutritional status and dialysis vintage). Read this blog post for a longer discussion about muscle mass and being black.
Regardless of the mechanism underlying higher serum creatinine, both the MDRD and CKD-EPI studies demonstrated a higher measured GFR in black persons for a given serum creatinine. This is hard to ignore.
But an argument could be made that in these studies race shouldn’t have been used as a variable. Racial categories are non-standardised and what the researcher documents as the race may not be congruent with what the patient self-reports. For more background on race in medicine and research see this JAMA Viewpoint article.
Adjusting for black race using the MDRD and CKD-EPI equations accounts for ~16% and ~18% increase in eGFR, respectively. But where might this become problematic? In nephrology, GFR 20 ml/min/1.73m2 and 30 ml/min/1.73m2 represent two watersheds around which important decisions regarding patient care are made. A slight over- or under-estimation of GFR in these regions could impact patient care in a number of ways.
Fig 1. Reimagining eGFR. from Eneanya et al, JAMA 2019.
The difference in CKD-EPI eGFR in the same self-identified black individual when considered black and non-black. The solid orange line represents the eGFR threshold for transplant referral in the US (<20ml/min/1.73m2) while the dashed blue line represents the eGFR threshold for referral to nephrology (<30ml/min/1.73m2).
The x-axis represents measured GFR, using urinary iothalamate clearance, from 534 individuals from the CRIC study.
The y-axis represents the difference between eGFR if non-black equation used (rather than black) for the same black participants. Hence the numbers are all positive, ranging from an eGFR that is higher, from about 2 to 14 ml/min/1.73m^2 or so.
Thus for participants clustering around the orange and blue lines, one can easily see how using the non-black equation can drop their GFR to make them qualify for referral.
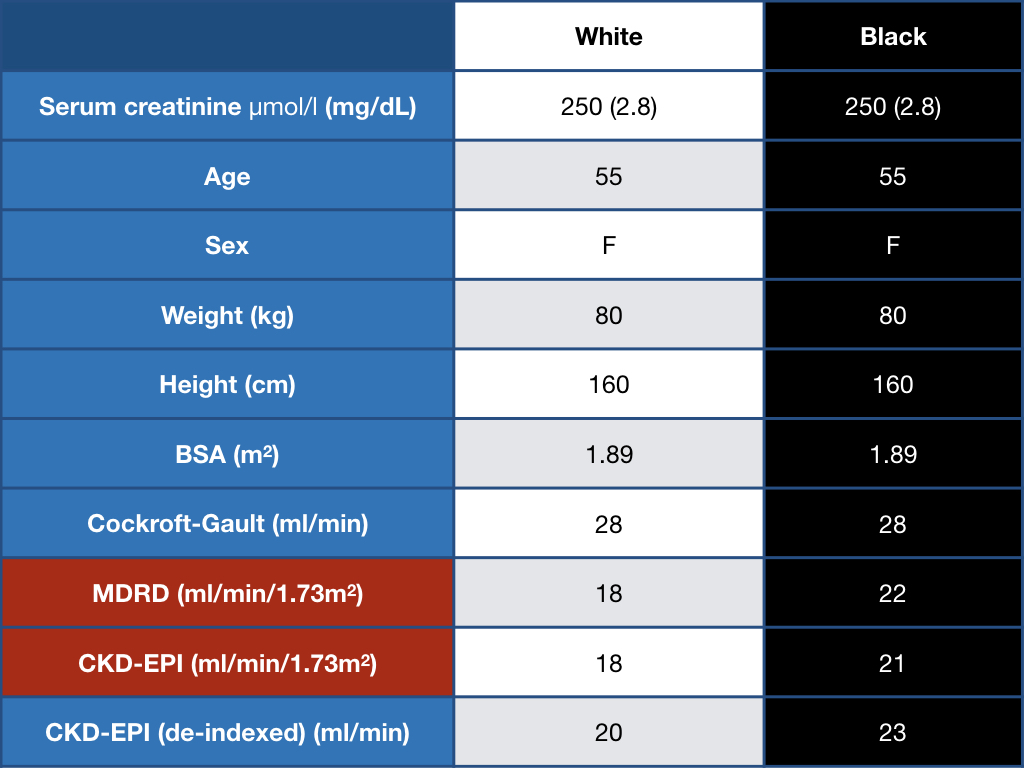
Look at this example worked out below. For a 55 year old woman with a serum creatinine of 2.8 mg/dL, a white woman would be referred for a transplant, a black woman would not.
Is identifying race easy?
Race was used as an easy heuristic - and in MDRD it was self-assigned. The CKD-EPI equation was derived from 10 different cohorts and validated in 16 different cohorts. But is identifying someone as black and white, excuse the pun, all that black and white? Are African-Americans the same as people of African ancestry from the Caribbean countries? Does black refer to people from sub-Saharan Africa only, or those from North Africa? How about the individuals from Somalia or Ethiopia? Can skin color be a proxy for making that assessment?
And what about individuals born of one black parent and one non-black parent - such as the past POTUS, and one of the current POTUS contenders. Should one look at skin color to make that assessment? Surely thats not very scientific.
Lastly, what of the rest of the world? Are these equations applicable to other populations from Asia, or Australia/New Zealand? Whether the race construct is valid or not, there may very well be differences in serum creatinine to GFR relationship in other parts of the world. Even in the CKD-EPI study, though there was better representation for blacks (around a third, up from the 197 participants in the MDRD study), Asians still constituted a very small proportion.
To address these, there are many different studies out there which have applied correction factors to the CKD-EPI equation for their own population. This study of 130 Indians (roughly half of whom were vegetarians) found that the CKD-EPI did overestimate GFR in that population. The CKD-EPI workgroup did do external validation of the equation, and published those findings in 2011. The study did include more populations from Asia - and report that measured GFR was 5% higher than estimated. The muscle mass story cannot explain this clearly, since no published data support higher muscle mass in Asians compared to whites. On the other hand, a Japanese study suggests their population has a GFR which is about 80% of that estimated from the CKD-EPI equation. Another study from Pakistan suggests the correction factor should be 0.686. Thus, studies from India, Japan and Pakistan suggest that the measured GFR is lower than estimated using the CKD-EPI equation for whites. This is in contrast to the CKD-EPI validation suggesting 5% higher measured GFR in Asians. Last we checked, India, Japan and Pakistan are still located within Asia. Interestingly, the 2011 validation paper by Stevens et al concludes with
‘..emphasis should be placed on investigation of filtration markers that may be less affected than creatinine by race and ethnicity, such as cystatin C and other novel markers. In summary, racial differences in performance of creatinine-based estimating equation likely reflect geographic and ethnic differences rather than race per se.’
Unintended Consequences
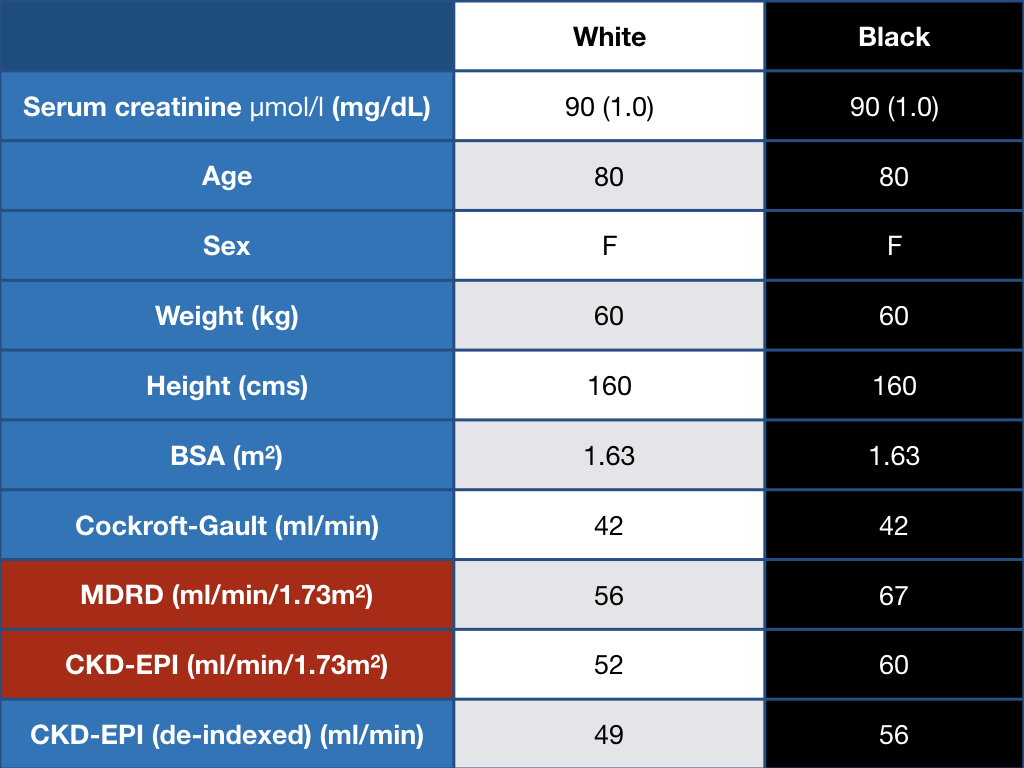
One can get rid of race - and use the same equation for everyone, however, that would be akin to throwing out the baby with the bathwater. We circle back to the fact that addition of the ‘race’ variable, as poorly defined as it is, seems to add more information, when estimating GFR and comparing it to measured GFR. Using the same (non-black version) of the equation would result in labelling a large swathe of the population as having CKD. Just see on example worked out below.
The way forward
Our estimation tools are far from perfect but we can make changes. Creatinine may vary by race - but cystatin C does not depend on muscle mass, so it should be less dependent on presumed race. There are other tubular markers studied in the past, perhaps they could make a comeback? Or should we actually measure GFR rather than estimate it - especially when decisions are being made, such as transplant referrals?
We can also make sure more care is taken if and when assessing race in future studies and we still have access to 24-hour creatinine clearance to remove the muscle mass question from the formula. Further research can be used to determine if eGFR calculations affect patient outcomes and access to certain interventions but even if the research is negative it still does not address the perception of care being racially biased. This article highlights a need for us to be cognizant of how we use race in our clinical practice and suggests that future eGFR calculation derivations should move beyond using race as a variable.
Summary by Ted J. FitzGerald,
NSMC Intern 2019
Nephrology Specialist Registrar,
Dublin, Ireland