#NephJC Chat
Tuesday March 13th 9 pm Eastern
Wednesday March 14th 8 pm BST, 12 noon Pacific
N Engl J Med. 2018 Mar 1;378(9):829-839. doi: 10.1056/NEJMoa1711584. Epub 2018 Feb 27.
Balanced Crystalloids versus Saline in Critically Ill Adults.
Semler MW1, Self WH1, Wanderer JP1, Ehrenfeld JM1, Wang L1, Byrne DW1, Stollings JL1, Kumar AB1, Hughes CG1, Hernandez A1, Guillamondegui OD1, May AK1, Weavind L1, Casey JD1, Siew ED1, Shaw AD1, Bernard GR1, Rice TW1; SMART Investigators and the Pragmatic Critical Care Research Group.
PMID: 29485925 for full text follow this link (till March 30, thanks to NEJM)
AND
N Engl J Med. 2018 Mar 1;378(9):819-828. doi: 10.1056/NEJMoa1711586. Epub 2018 Feb 27.
Balanced Crystalloids versus Saline in Noncritically Ill Adults.
Self WH1, Semler MW1, Wanderer JP1, Wang L1, Byrne DW1, Collins SP1, Slovis CM1, Lindsell CJ1, Ehrenfeld JM1, Siew ED1, Shaw AD1, Bernard GR1, Rice TW1; SALT-ED Investigators.
PMID: 29485926 for full text follow this link (till March 30, thanks to NEJM)
NEJM Video Summary
Also check out the Editorial in NEJM, and early reactions from across the FOAMed Community:
Josh @pulmcrit likes it and is convinced we should switch to balanced solutions
Rory @EMNerd_ is not, and points out his misgivings about the surrogate outcome
Some tweets from SPLIT PI Paul Young
Introduction
The modern version of this story begins with a before/after study which broke into JAMA a few years ago. Conducted in the ICU in a university affiliated hospital in Melbourne almost a decade ago, all patients received normal saline as the resuscitation fluid of choice, followed by a 6 month phase out period, and then 6 months of a balanced solution (either Hartmann's, Plasma-Lyte 148 or chloride-poor albumin). See how these solutions differ:
*figures refer to concentration in mmol/L. Hartmann and ringer were two different individuals who came up with similar formulations. Actual concentration may vary a bit based on manufacturer.
Yunos et al reported a fantastic reduction in the incidence of AKI (defined by RIFLE criteria, OR 0.52) and need for RRT (OR 0.52) with no significant effect on ICU stay of mortality. Not a randomized study, so possibly Hawthorne effect and other biases, the skeptic might point out (check this convo on PBFluids). Fast forward a few years, another study from the Southern Hemisphere, this time from New Zealand. In contrast to the Yunos study, SPLIT was a randomized, blinded trial. And it did not report a significant difference in the risk for AKI, covered here for #NephJC. Check out Joel's palpable disappointment here. So how do we resolve this impasse? More trials - Vanderbilt to the rescue now, with two trials: SMART and SALT-ED. SALT-ED included patients seen in the Emergency Department, who received more than 500 ml intravenous fluids, and did not get admitted to the ICU. SMART included patients admitted to the ICU, whether from the Emergency or from the Operating rooms. Lets see what else was planned in these two trials.
The Studies
Methods
These are both
single centre
pragmatic
unblinded
cluster randomized
multiple cross-over (by month)
interventional trials
SALT-ED enrolled patients in the Emergency department, who received at least 500 ml crystalloids, and were hospitalized, over a period of 16 months (so alternating 8 cycles of saline and balanced crystalloids).
SMART included all patients admitted to any of 5 ICUs, over a varying period of time (see figure below, or figure S1 in supplementary data), again with alternating months of saline and balanced crystalloids.
The pragmatic aspect refers to the fact that the inclusion/exclusion criteria are not very detailed - all patients got included (unless they received < 500 ml of crystalloids and were sent home, in SALT-ED). Secondly, to enroll all such patients, another key aspect was waiver of patient consent. The last aspect of the pragmatism is the use of the electronic order entry to ensure the patients received the correct intervention. This guided the ordering provider through relative contraindications to balanced solutions (including brain injury, but also strangely hyperkalemia [no exact value given and was up to discretion of clinician]). See some comparisons with the other studies in table below:
Outcomes and Analysis
This deserves some attention. So don't skip this over!
SALT-ED: Primary outcome was hospital-free days to day 28 (which itself is a composite of in-hospital death and hospital length of stay). So worst case would be if someone who either died in first 28 days, or was not discharged for 28 days. There were 3 'key' secondary outcomes:
Major Adverse Kidney Events (MAKE) within 30 days: which was a composite of death, new renal replacement therapy or persistent kidney dysfunction. This last event was defined as a final serum creatinine > 200% of baseline value.
Stage 2 AKI or worse (KDIGO criteria)
Death in-hospital
Patients already on dialysis were enrolled in the trial - but they could only meet secondary outcome by dying. The baseline creatinine does not refer to admitting/initial creatinine, since a patient may already be in AKI at presentation. It refers to the creatinine in the year before presentation in the EMR. If not available (as was the case for about 35%), it was imputed as: 0.74 – 0.2 (if patient is female) + 0.08 (if patient is black) + 0.003 × age (in years)
SMART: The primary outcome here was MAKE at hospital discharge or 30 days (whichever came first). MAKE was defined as above. The baseline creatinine was also calculated as above. The secondary outcomes included
in-hospital death before ICU discharge or at 30 days or 60 days
ICU-free days, ventilator-free days, vasopressor-free days, and days alive and free of RRT within 30 days
Secondary renal outcome included new RRT, persistent renal dysfunction (as above), or AKI stage 2 or worse (KDIGO), the highest creatinine during hospital stay, and change in creatinine (highest to baseline, final creatinine)
Sample Size & Analytic Plan
SALT-ED: The trial was powered for a 0.5 day difference in the primary outcome (hospital-free days). Based on historical data of the number of patients, 14,000 patients could be enrolled in 16 months, with the saline group having a 24 (+/-) 4 days hospital free days.
SMART: The assumption here was a 22% event rate in saline group, which would entail enrolling 8000 patients (in about 60 unit-months). However, they checked their own observational data, and saw a lower event rate (~ 15%) so they bumped up the enrolment to 14,000 patients and 82 unit-months.
Results
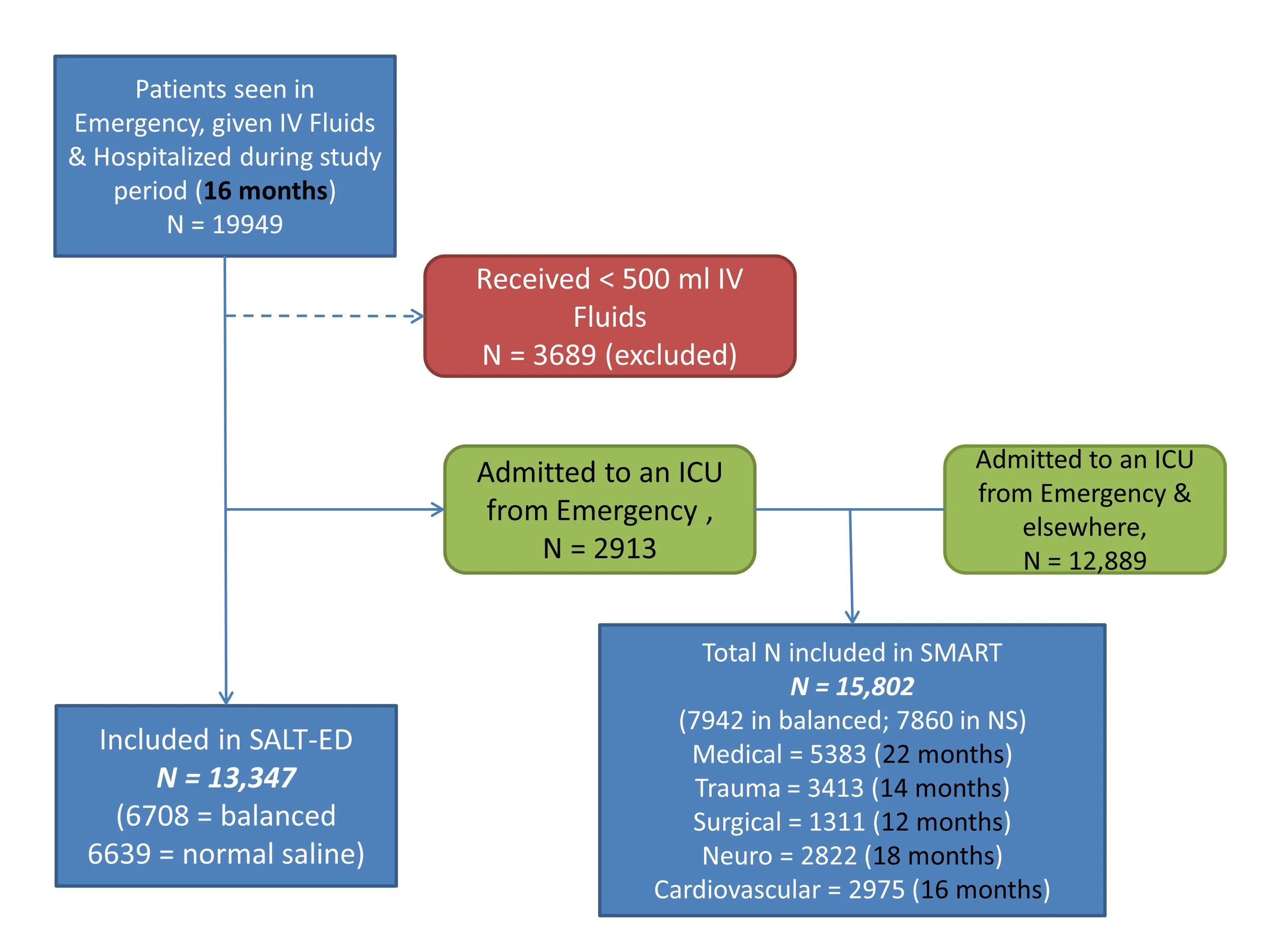
Enrolment happened as follows:
Composite flow diagram created from supplementary data. Not explicitly stated, but if SALT-ED ran for 16 months, and SMART duration varied depending on the ICU, as stated in parenthesis, presumably more than 2913 patients included in SMART were from the Emergency department. eg most Trauma patients (N = 3413) would be from the Emergency, which is greater than N = 2913 which are mentioned in SALT-ED
So they did get the roughly 14,000 patients they wanted in each trial. For the characteristics, see some key aspects from the table 1 below
1. The risk scores refers to APACHE II for Yunos et al and SPLIT
2. Predicted risk of in-hospital death is an estimated probability of death before hospital discharge generated through the Vizient database
3. The Elixhauser Comorbidity Index score summarizes the burden of a patient’s coexisting conditions. Scores range from −19 to 89, with higher scores indicating a profile of coexisting conditions that is more strongly associated with in-hospital death
4. Initial = first creatinine at emergency visit; baseline = from last 1 year, imputed for ~35%
Most patients did get the crystalloid they were supposed to get.
And what happened?
The chloride and bicarbonate changes did happen as expected
Change in bicarb and chloride. Modified from Figure 1 (SALT-ED, over 72 hours) and figure 2 (SMART, over 7 days)
The primary outcome in SALT-ED was hospital-free days. This was not significantly different between groups. The secondary outcome of MAKE, however, was significant (with numerically lower events with balanced solutions in all 3 components of MAKE, table 3).
For SMART, the composite primary outcome, MAKE, was significant, with lower deaths and new RRT pushing the difference (table 2). See the table below to see how the individual events compare with Yunos et al and SPLIT.
Comparison of clinical outcomes.
1: AKI refers to injury + failure (RIFLE) for Yunos et al; KDIGO stage 2 or higher in SPLIT, SMART & SALT-ED
2: Change in creatinine is highest value/peak value and initial creatinine. Initial creatinine = baseline creatinine, calculated differently for SMART/SALT-ED as above.
3: refers to ICU-free days in first 28 days
4: refers to hospital free days in first 28 days
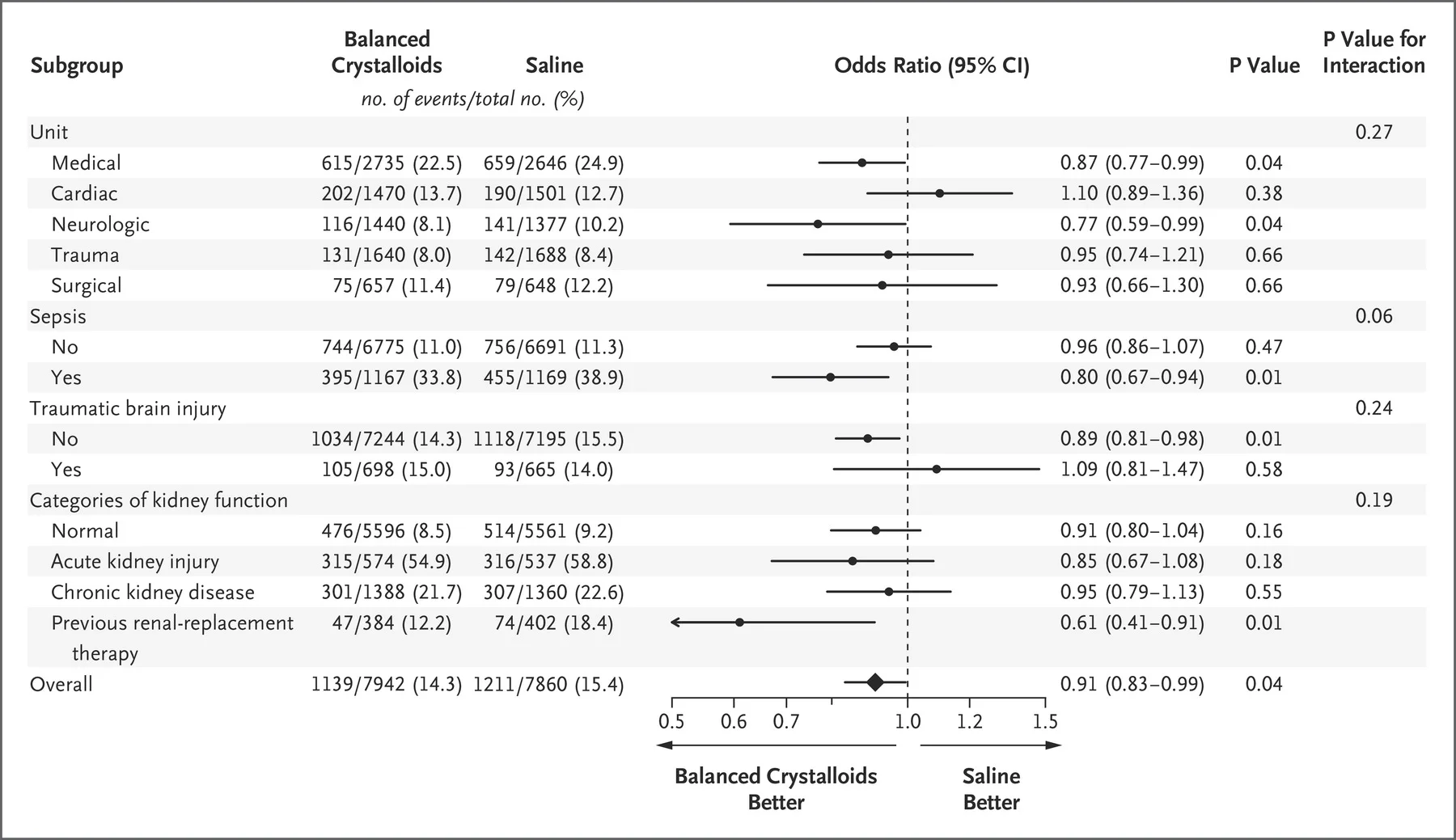
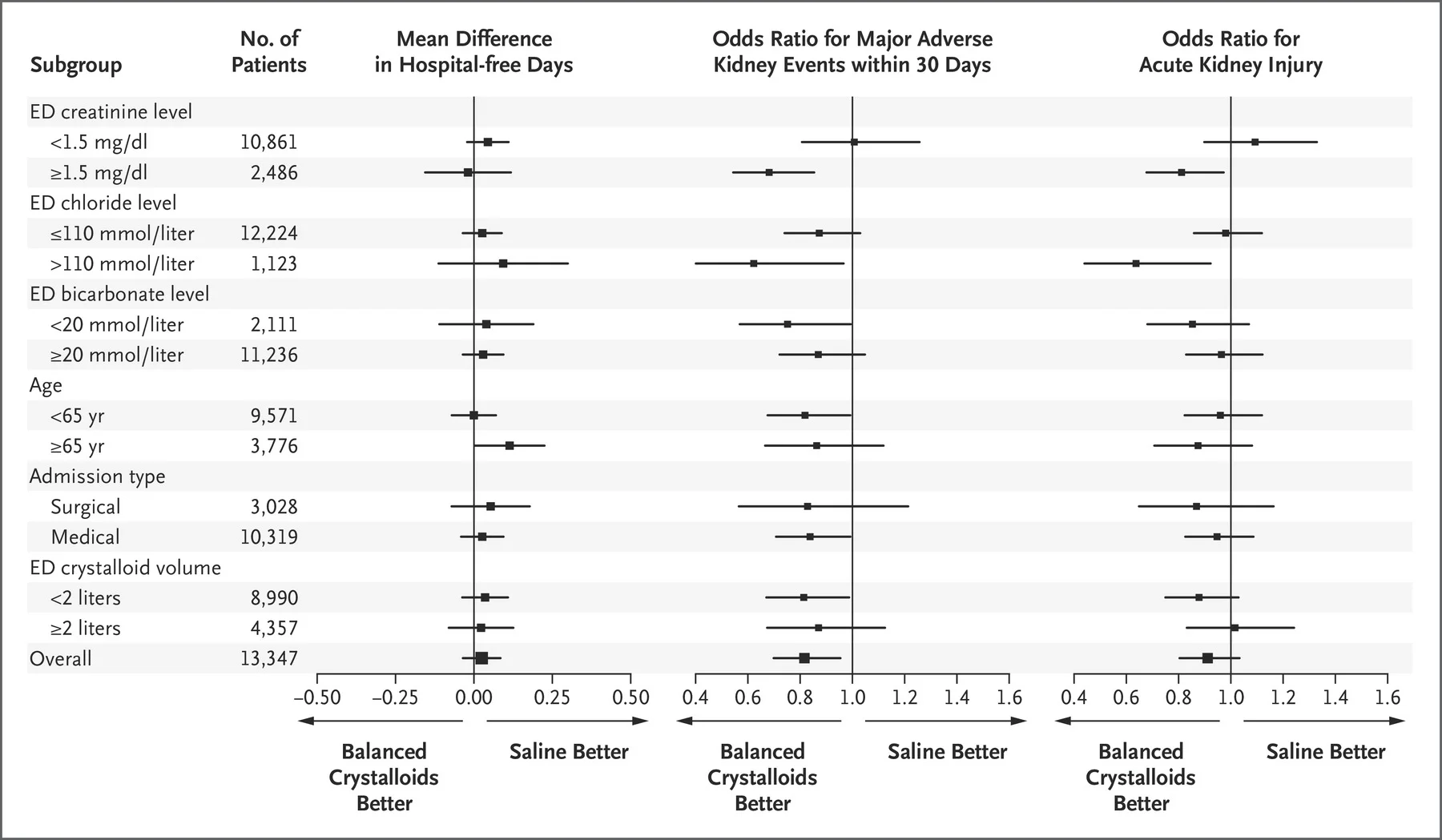
Subgroup Analysis
A bunch of these were done. None of the interaction values are significant (though not reported below for SALT-ED)
SMART
Figure 3 from Semler et al, NEJM 2018
SALT-ED
Figure 2 from Self et al, NEJM 2018
Sensitivity and Per Protocol analysis
In addition to the analysis above, a per protocol analysis done was done on the basis of fluids administered (since there was a small amount of crossover) with no difference observed. Given the pragmatic nature, and many assumptions (eg those baseline creatinine calculations) - sensitivity analyses excluding these patients are presented (and they don't change the thrust of the main results). In addition, for SMART, since patients could be enrolled twice for re-admissions, or they could receive both interventions if they stayed longer than 1 month, sensitivity analyses excluding those patients were done, with no difference again seen. Lots of these results are presented in the detailed supplements. Worth perusing.
Discussion
Strengths
Large sample size. Indeed, largest trials on this topic so far!
Pretty good adherence to the assigned crystalloid
Important outcomes.
The results are coherent: even the non-significant results have consistent point estimates, and the benefit in the lower risk SALT-ED population is lower than in the higher risk SMART population
One could argue effect is small - but considering the amount of IV Fluids given, the difference if true is truly very significant clinically at a population level
Limitations
Lack of blinding (though how would that have an effect on the outcome?)
Lack of allocation concealment (ie one would know what IV fluid would be used tomorrow if a patient is enrolled). But again, as above, it is hard to make a case for how this would be actually bias the results.
Importance of outcome: the positive outcomes seem to be mostly driven by persistent kidney dysfunction on the basis of the difference from baseline
The amount of fluids given appear to be quite small to have such an effect?
So should we switch over to using balanced solutions? What explains the difference between these results versus the smaller, but perhaps better designed SPLIT? The volumes of IVF were similar - could something about the solution itself (Plasmalyte versus Ringer's) explain the difference? There is little downside to using balanced solutions - a small cost difference of a few cents, and there is no signal of balanced solutions being worse. Or do you want to wait for the randomized and blinded trial of higher risk patients (N = 8300) aka the PLUS trial? Or the FLUID trial, N = 65,000, another cluster RCT? Or maybe the BEST FLUID trial, specifically aimed at DGF? Or the even larger BASICS trial, from Brazil? Join us to discuss
Summary by Swapnil Hiremath, Ottawa