#NephJC Chat
Tuesday, February 9th, 2026, 9 pm Eastern on Bluesky
Clin J Am Soc Nephrol. 2025 Dec 12. doi: 10.2215/CJN.0000000951. Online ahead of print.
Efficacy, Mechanisms, and Safety of Sodium-Glucose Cotransporter-2 Inhibitors in Kidney Transplant Recipients: A Randomized, Double-Blind, Placebo-Controlled Trial
Vikas S Sridhar, Luxcia Kugathasan, Yuliya Lytvyn, Hongyan Liu, Yangqing Deng, Leif Erik Lovblom, Massimo Nardone, Darren A Yuen, Yixiao Chen, Jonathan Hua, Yarden Aronson, Mai Mohsen, S Joseph Kim, Jacob A Udell, Bruce A Perkins, Jasper Stevens, Daan J Touw, Hiddo J L Heerspink, David Z I Cherney, Sunita K S Singh
PMID: 41385300
Introduction
“Life is not about finding our limitations; it’s about finding our infinity”
Few would argue against kidney transplantation being the best choice for renal replacement therapy in terms of patient well-being and survival. Acute and subacute rejection have been addressed through effective immunosuppressants, with 1-year graft survival rates reaching 98%, but there remains a real need to tackle long-term complications and particularly progressive eGFR loss in complex transplant patients (Davis et al, CJASN 2022). The main reasons for graft failure include: death from cardiovascular disease, worsening CKD caused by immune system damage (chronic allograft injury/nephropathy), and recurrence of the native kidney disease (i.e., diabetic nephropathy). Because of these insidious processes, 10-year survival in kidney transplantation recipients only reaches roughly 50% (Hariharan et al, NEJM 2021). Furthermore, post-transplantation serum creatinine renal function at 1 year post-transplantation predicts long-term graft survival; a 0.5 mg/dL increase in serum creatinine can significantly affect long-term renal graft survival (Hariharan et al, Kidney Int 2002). With the advent of the four pillars of CKD care (SGLT2i, GLP-1 RA, nsMRA, RASi), there has been a reduction (or even a remission) of eGFR loss in pre-transplant CKD patients. However, the role of most of these agents in kidney transplantation is not well defined, and there is an urgent need to explore the efficacy and safety of each of these agents in transplant patients. Kidney transplant patients possess a higher risk for CVD, compared to the general population, due to the frequent presence of traditional risk factors (i.e., diabetes 24-40%, hypertension 40-90%, dyslipidemia 50%, smoking 25%) and nontraditional risk factors (alloimmunity, adverse metabolic effects of immunosuppressants, chronic anemia, chronic inflammation, proteinuria, and hyperhomocysteinemia) (Rangatswami et al, NDT 2019 and Lam et al, Kid Int 2017). In addition, glomerular cellular stress (podocyte and endothelial) from glomerular hypertrophy can result in proteinuria and allograft failure (Menon et al, Kidney Int 2022). Sodium glucose transporter inhibitors exhibit pleiotropic effects that provide survival advantages, as seen in multiple studies of cardiovascular and kidney outcomes. Though the exact mechanisms are unclear, and glucose-lowering effects alone do not fully explain them, it is probable that natriuresis, osmotic diuresis, and reduced glomerular hypertension contribute to the plethora of clinical benefits. The question remains: do these findings and resulting benefits carry over into patients who have received a kidney transplantation?
The Study
Methods
Study participants
INFINITI (NCT04965935) was a mechanistic, randomized, double-blind, placebo-controlled trial in kidney transplant recipients.
Study design
Initially limited to participants with diabetes, eligibility was later expanded to non-diabetic patients, following publication of DAPA-CKD study. Participants were randomized 1:1 to dapagliflozin 10 mg or placebo. Assessments were performed at baseline, week 1, and week 12. Allocation was pharmacy-controlled, and all investigators and participants were blinded. Physiologic and mechanistic assessments were performed at three prespecified timepoints: baseline, week 1 (acute drug effects), and week 12 (for chronic exposure).
Supplemental Figure 1. Study design for INFINITI from Sridhar et al, CJASN 2025
The outcomes were mechanistic in scope, designed to characterize the physiologic effects of SGLT2 inhibition in kidney transplantation. The primary endpoint was seated systolic blood pressure. Secondary outcomes mapped renal and systemic physiology.
Integrated physiologic assessment platform
Hemodynamic and vascular assessment
Blood pressure was measured after rest using an automated device, with seated and supine assessments. Systemic hemodynamics (cardiac output, stroke volume, and vascular resistance) were evaluated using noninvasive cardiac output monitoring. Arterial stiffness was assessed by applanation tonometry, including augmentation index and pulse wave velocity.
Autonomic and anthropometric profiling
Cardiac autonomic tone was evaluated through heart rate variability analysis. Anthropometric measures, including body weight, were recorded, and estimated plasma volume was calculated to contextualize volume status.
Kidney function and metabolic assessment
Kidney function was quantified using measured GFR (mGFR) by iohexol plasma clearance, providing a direct assessment of filtration independent of creatinine kinetics. Blood samples for eGFR using CKD EPI 2021 were collected in parallel for clinical comparability. Fasting metabolic parameters, including plasma glucose, were obtained at each study visit.
Tubular sodium handling assessment
Segment-specific tubular sodium transport was evaluated using a lithium clearance technique. Participants received oral lithium carbonate the evening prior to testing, followed by fasting blood urea and urine collection the next morning. Fractional excretion of lithium (FELi) was used to estimate proximal tubular sodium delivery and reabsorption, while fractional excretion of sodium (FENa), interpreted alongside FELi, provided information on distal sodium handling. This approach allowed mechanistic evaluation of the effects of SGLT2 inhibition beyond global natriuresis.
Statistics
The trial was powered for the primary endpoint of change in seated systolic blood pressure, assuming an approximate 6.5 mmHg between-group difference with a target sample size of 52 participants. Analyses were conducted under the intention-to-treat principle. Longitudinal outcomes were analyzed using linear mixed-effects models including fixed effects for treatment, time, and treatment x time interaction, with a participant-level random intercept.
Missing data were handled under a missing-at-random assumption using restricted maximum likelihood estimation. Secondary and mechanistic outcomes were exploratory and not adjusted for multiple comparisons. Prespecified subgroup analyses were performed by diabetes status.
Funding
The trial was investigator-initiated and supported by academic funding sources. Study drug and/or logistical support were provided by the manufacturer of dapagliflozin. The funders had no role in trial design, data analysis, or interpretation.
Results
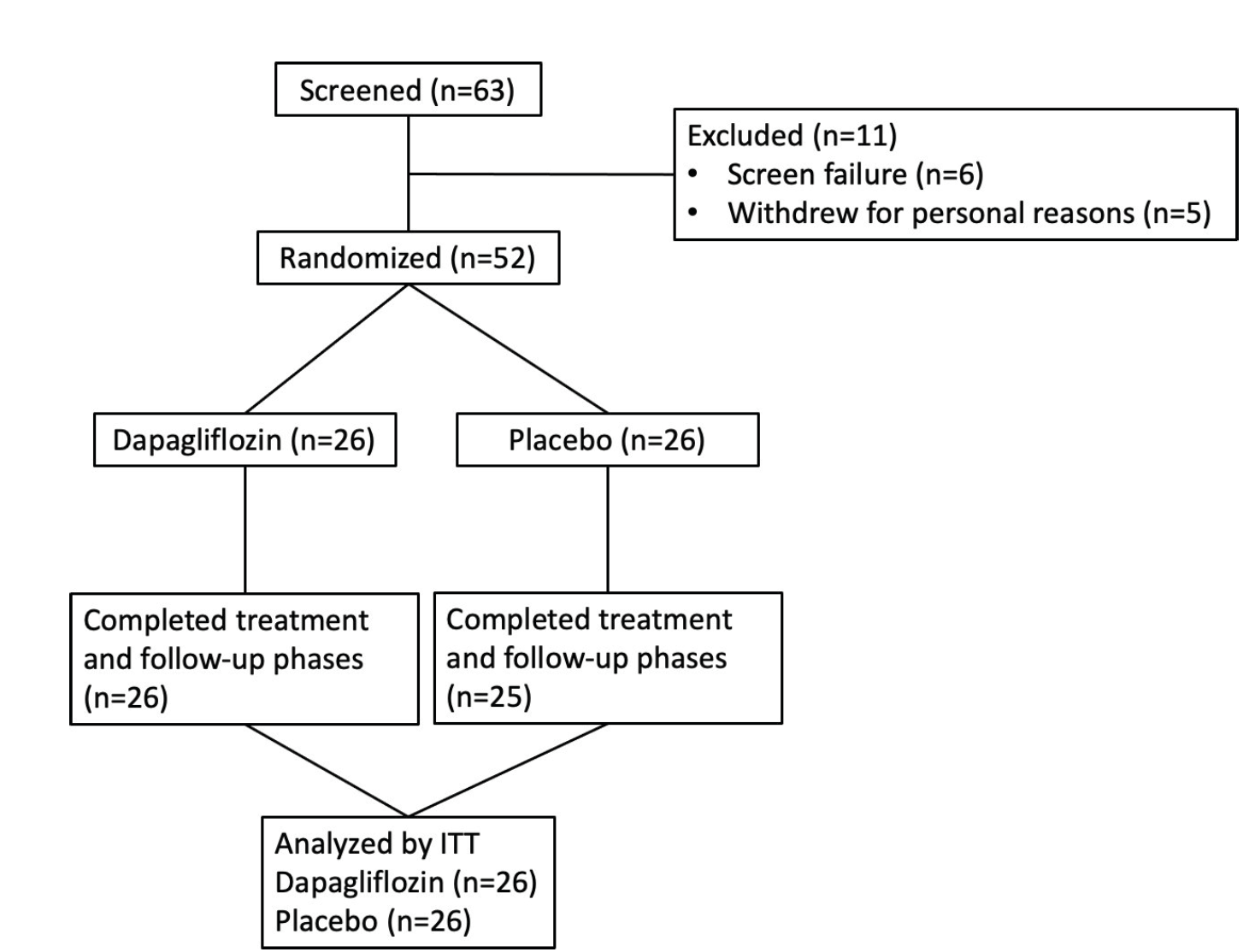
INFINITI enrolled 52 stable kidney transplant recipients, more than 6 months post-transplant, with reasonably preserved graft function, and 51 patients completed the study.
Supplemental Figure 2. Study participant flow diagram, Sridhar et al, CJASN 2025
Baseline characteristics
The mean age of the participants was 53 years. Comorbidities were common with 57% diabetics, 62% with hypertension. Half of study participants were receiving renin angiotensin aldosterone system inhibitors (RAASi) at baseline, and most were on tacrolimus based immunosuppression. Mean baseline eGFR was 68 ml per minute per 1.73 m². Baseline characteristics were well balanced between the dapagliflozin and placebo groups (table 1).
Table 1. Baseline demographic and clinical characteristics at randomization in kidney transplant recipients stratified by dapagliflozin or placebo, from Sridhar et al, CJASN 2025
Primary endpoint: blood pressure
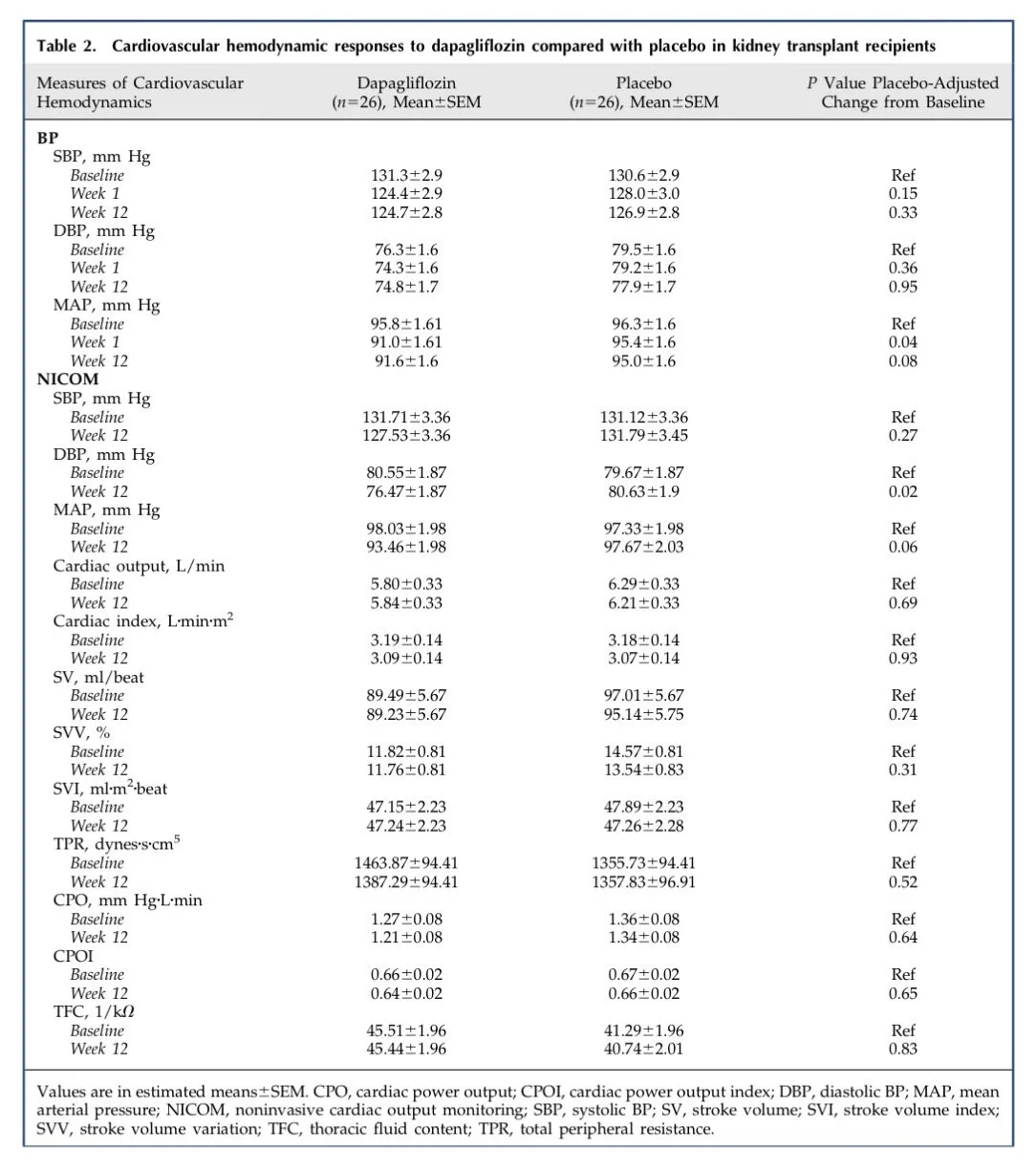
The primary endpoint of the trial was systolic blood pressure. Dapagliflozin did not reduce systolic BP at one week or at twelve weeks compared with placebo.
There was a small early drop in mean arterial pressure at one week with a placebo adjusted reduction of 3.9 mmHg. This effect did not persist at twelve weeks and did not translate into sustained systolic BP lowering. Diastolic blood pressure did not differ between groups across timepoints.
Table 2. Cardiovascular hemodynamic responses to dapagliflozin compared with placebo in kidney transplant recipients from Sridhar et al, CJASN 2025
Kidney function
One of the most convincing signals in INFINITI was the change in mGFR. Using iohexol clearance, dapagliflozin caused a statistically significant reduction in mGFR at both 1 and 12 weeks. The placebo-adjusted decline was -4.2 ml per minute per 1.73 m² at one week and -3.5 ml per minute per 1.73 m² at twelve weeks. Decline in eGFR showed similar directional changes, mirroring the early hemodynamic dip seen in native kidney SGLT2 inhibitor trials. Importantly, this fall in GFR was not accompanied by biochemical instability or clinical acute kidney injury.
Table 5. Kidney responses, from Sridhar et al, CJASN 2025
Tubular sodium handling and volume parameters
Dapagliflozin significantly increased urinary glucose excretion at one week and twelve weeks. 24-hour urine volume also increased and remained elevated at both timepoints.
Table 4. Sodium handling response, from Sridhar et al, CJASN 2025
Fractional sodium excretion, fractional excretion of lithium, and derived distal sodium handling indices did not differ between dapagliflozin and placebo at week 1 or week 12. Measured 24-hour urinary sodium excretion was unchanged, and estimated plasma volume did not differ between groups.
Vascular and hemodynamic measures
Measures of arterial stiffness, including augmentation index and pulse wave velocity, did not show significant numerical reductions in the dapagliflozin group at twelve weeks. Non-invasive cardiac output measurements also did not differ between groups. Plasma renin, aldosterone, catecholamines, heart rate, and heart rate variability did not differ between the dapagliflozin and placebo groups at one week or twelve weeks.
Table 2. Cardiovascular hemodynamic response, from Sridhar et al, CJASN 2025
Metabolic parameters
Dapagliflozin was associated with reductions in body weight and body mass index at one week and twelve weeks compared with placebo. Fasting glucose, HbA1c, and lipid parameters did not differ significantly between groups. Serum bicarbonate was higher in the dapagliflozin group at week 12 (supplemental table 5).
Safety
Dapagliflozin was well tolerated. There were no cases of urinary tract infection or genital infection in either group. Rates of hypoglycemia, volume depletion, acute kidney injury, and rejection episodes were low and similar between groups. Tacrolimus levels remained stable throughout the study.
Supplemental Table 6. Adverse events in the INFINITI trial, from Sridhar et al, CJASN 2025
Discussion
The number of kidney transplant surgeries done annually is on the rise. As patients benefit from kidney transplants and live longer and healthier lives, the specter of CKD often re-emerges over time. Now, with newer medications for the treatment of CKD, the challenge is when and how to introduce them to a kidney transplant population. It is understood, and highly probable, that not every class of medication for CKD patients will be efficacious or safe for kidney transplant patients. For example, in one small RCT from New Zealand, treatment with ramipril compared with placebo did not lead to a significant reduction in doubling of serum creatinine, end-stage renal disease, or death in kidney transplant recipients with proteinuria (Knoll et al. Lancet 2016). Newer therapies need to be systematically studied to address cardio-kidney metabolic abnormalities for outcomes in post-renal transplant patients.
The INFINITI study seeks to address one of these newer classes of medications. Physicians prescribing SGLT2i for kidney transplant recipients who have CKD need to consider: a) the immunosuppressed state of patients, and b) the impact of SGLT2i on denervated kidneys, and c) the potential physiologic interactions of SGLT2i with calcineurin inhibitors.
A meta-analysis in nontransplant patients showed that though the SGLT2i use is not associated with increased risk of UTI, it is associated with genital mycotic infections (Puckrin et al, Acta Diabol 2018). It is likely that this risk will be even greater in immunosuppressed patients. It will be important, on an individualized basis, to consider an individual’s infection risk, and decisions about initiation should include shared decision-making.
Figure from Puckrin et al, Acta Diabol 2018
Table prepared from supplementary material Sridhar et al, CJASN 2025
INFINITI did not meet its primary endpoint of reducing seated systolic blood pressure. However, modest but significant reductions in mean arterial pressure and supine diastolic blood pressure were observed early and persisted over 12 weeks. The magnitude of these reductions, approximately 3–5 mmHg, aligns with reductions reported in major SGLT2 inhibitor trials in non-transplant populations, yet the signal was weaker and inconsistent across blood pressure measures. Crucially, mechanisms typically implicated in SGLT2-mediated blood pressure lowering were largely absent: no consistent improvement in arterial stiffness, no reduction in systemic vascular resistance, and no evidence of sympathetic nervous system suppression. This suggests that transplant-specific vascular and tubular physiology may blunt the hemodynamic response to SGLT2 inhibition, potentially related to calcineurin inhibitor–induced sodium retention and vascular tone changes.
One of the most clinically important observations was the clear acute decline in measured GFR following dapagliflozin initiation, sustained at 12 weeks. The magnitude of this dip closely mirrors what has been seen in other non-transplant cohorts. This strongly supports preservation of intraglomerular hemodynamic unloading in transplanted kidneys, a mechanism thought to underlie long-term nephroprotection. Notably, this occurred without significant albuminuria reduction, reinforcing emerging data that the benefits of SGLT2 inhibitors extend beyond proteinuria lowering.
One of INFINITI’s most intriguing findings was what didn’t happen: despite strong glucosuria, higher urine volume, and a preserved GFR dip, there was no meaningful increase in proximal sodium excretion. This challenges the classic SGLT2i story in which natriuresis drives tubuloglomerular feedback and intraglomerular pressure reduction. Possible explanations include rapid distal sodium reabsorption, calcineurin inhibitor–related sodium retention, and a growing recognition that SGLT2i benefits may rely more on hemodynamic unloading and tubular energetics than on sustained sodium loss.
Strengths: This randomized trial benefits from rigorous kidney function assessment using iohexol-measured GFR and provides mechanistic insight into tubular sodium handling through lithium clearance. The study design supports internal validity, and the intervention appears safe and feasible in the studied population.
Limitations: The main limitations include short follow-up, and inclusion of a predominantly low-risk population, which may limit generalizability. Given the small number of patients, it is difficult to say anything about sub-groups (of note, women and minorities were underrepresented). Also, residual confounding from concomitant medications cannot be excluded.
Ongoing studies: There is an ongoing randomized, placebo-controlled clinical trial (the RENAL LIFECYCLE Trial; ClinicalTrials.gov NCT05374291) investigating the effects of dapagliflozin 10 mg daily compared with placebo in patients with advanced chronic kidney disease (eGFR ≤ 25 ml/min/1.73 m²), including those on dialysis and kidney transplant recipients with an eGFR ≤ 45 ml/min/1.73 m², to assess its impact on kidney failure, heart failure-related hospitalization, all-cause mortality, and safety in this high-risk population.
Conclusion
The INFINITI study showed that SGLT2 inhibitors were safe and preserved their protective hemodynamic “GFR dip” in kidney transplant recipients, but with altered physiology, including muted natriuresis and BP effects. Long-term studies with hard outcomes (i.e., time to return to dialysis or retransplantation) are needed to confirm clinical benefits.
Summary by
Sai Vani Yellampalli
Akshaya Jayachandran
Bogdan Agavriloaei
Reviewed by
Milagros Flores, Pallavi Prasad, Cristina Popa, Brian Rifkin








Putting the mellitus back into diabetes. Is inducing glycusuria the key to diabetic nephropathy? EMPA-REG renal outcomes.