#NephJC Chat
Tuesday February 25, 2020 at 9 pm Eastern
Wednesday February 26, 2020 at 9 pm Indian Standard Time
Wednesday February 26, 2020 at 9 pm GMT
N Engl J Med, 382 (7), 622-631 2020 Feb 13
Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis
Michael Walsh, Peter A Merkel, Chen-Au Peh, Wladimir M Szpirt, Xavier Puéchal, Shouichi Fujimoto, Carmel M Hawley, Nader Khalidi, Oliver Floßmann, Ron Wald, Louis P Girard, Adeera Levin, Gina Gregorini, Lorraine Harper, William F Clark, Christian Pagnoux, Ulrich Specks, Lucy Smyth, Vladimir Tesar, Toshiko Ito-Ihara, Janak Rashme de Zoysa, Wojciech Szczeklik, Luis Felipe Flores-Suárez, Simon Carette, Loïc Guillevin, Charles D Pusey, Alina L Casian, Biljana Brezina, Andrea Mazzetti, Carol A McAlear, Elizabeth Broadhurst, Donna Reidlinger, Samir Mehta, Natalie Ives, David R W Jayne, PEXIVAS Investigators
PMID: 32053298 Full Text at NEJM
Editorial in NEJM
NephMadness 2019 Scouting Report on Plasma Exchange
Introduction
Yes, one should do large randomized controlled trials (RCTs) whenever possible. However ANCA vasculitis is not a common disease, so can one even try to do a large RCT? As a wise person once said, do, or do not, there is no try. So the PEXIVAS Investigators went ahead and performed the largest RCT in ANCA Associated Vasculitis (AAV) ever. These ground-breaking results may change clinical practice in nephrology.
How big is the problem? If left untreated, AAV is associated with 80% mortality at 1 year, approaching 100% within 5 years. With current induction regimens, including cyclophosphamide and rituximab, AAV is no longer a rapidly fatal disease but one of chronic morbidity and reduced long term survival, often preceded by ESKD. Unselected cohorts often demonstrate 5-year kidney survival (defined as the composite of ESKD or death) of 60 to 70%. Unfortunately some of the morbidity and mortality these patients suffer is due to high dose of immunosuppression that comes with contemporary therapy.
To understand the therapy targets, remember that ANCA causes the development of vasculitis through several proposed mechanisms, some of which were covered in the last NephJC.
Can you mention more than 10 trials in AAV? It is ok if you don’t remember, we have your back! The current trials of immunosuppressive therapy were summarized by one of our 2020 NSMC Interns, Namrata Parikh in a meticulous tweetorial of AAV induction and maintenance therapy, and the curious reader can also check out the landmark trials blog post on AAV.
Evolution of Therapy: Soon after steroids were discovered and made available for pharmaceutical use in the 1950s, they were utilized in vasculitis (ANCA were described much later, in the early 1980s). In 1967, Frohnert and Sheps described their experience with 110 patients who had received corticosteroids, with a 5 year survival of 48% (compared to 14% in those who did not get steroids, pretty much following the ghastly natural history).
The next major advance was cyclophosphamide. In 1979, Fauci and colleagues reported fantastic results with cyclophosphamide, with 14 of 17 patients with severe necrotizing vasculitis surviving and entering remission, with no relapses. Note: both steroids and cyclophosphamide were case series, but the results were so dramatic, they didn’t need any RCTs to enter into clinical use. Not so much for the attempts that followed, however.
We won’t discuss Rituximab much, but one can read the RITUXVAS and RAVE trials for more. They did establish rituximab as an acceptable alternative to cyclophosphamide (though not necessarily superior) for induction of ANCA vasculitis.
But given the ANCA are antibodies, shouldn’t removing the antibodies provide faster remission, as it seems to do in anti-GBM disease? Surely it would? That lead to the MEPEX trial, which compared 3g of IV methylprednisolone to 7 sessions of plasma exchange (PLEX) in 140 patients. Both groups received oral cyclophosphamide and prednisolone after the randomized therapies. PLEX decreased progression to ESKD by 24% (with a fragility index of 5), but there was no difference in death at 1 year, or serious adverse events. A subsequent meta-analysis with 378 patients from 9 RCTs expanded on these results, showing a reduction in ESKD (RR 0.64, 95% CI 0.47 to 0.88), but not mortality (RR 1.01, 95% CI 0.71 to 1.41). For the combined ESKD/death outcome it was an even closer call, with an RR of 0.80 (95% CI 0.65 to 0.99). That doesn’t engender a lot of confidence in the superiority of removing ANCA.
Additionally, high dose steroids have remained the mainstay of therapy throughout this period. Perhaps, with the addition of these efficacious and powerful agents for induction and maintenance, giving lower dose of steroids may be good enough to achieve benefit without adverse effects ?
Why was PEXIVAS designed?
Apart from the considerations discussed above, approximately 30% of patients with AAV either do not have adequate disease control or are intolerant of their induction of remission treatment. An additional 50% of patients will have relapsing AAV over the subsequent five years.
Inadequate disease control is associated with increased immunosuppressive medication and thus increased risk for treatment-related toxicity and progressive organ scarring and death. Additionally, between 25 and 50% of patients with severe AAV experience a severe infection within the first 12 months of treatment and the most frequently cited causes of death are infection or uncontrolled vasculitis.
The Study
Methods
Study Design
PEXIVAS included patients with severe, active ANCA-associated vasculitis with a 2-by-2 factorial design, which allowed separate evaluations of initial treatment with plasma exchange as compared with no plasma exchange (with either cyclophosphamide or rituximab administered to all patients) and of two different regimens of oral glucocorticoids. (Read more about 2x2 factorial designs here). For the comparison of PLEX versus no PLEX, the hypothesis was that PLEX would be superior, whereas for the two steroid arms, the hypothesis was that lower dose steroids would be non-inferior (read more on non-inferiority design here). The randomization was performed with the use of a minimization algorithm, with stratification according to age, kidney function, ANCA subtype, severity of pulmonary hemorrhage and planned type of induction immunosuppressive therapy.
Population
Inclusion Criteria:
15 years of age or older,
New or relapsing AAV (either GPA: granulomatosis with polyangiitis or MPA: microscopic polyangiitis)
Positive MPO or PR3 antibodies (by ELISA)
Kidney involvement (with an estimated glomerular filtration rate of less than 50 ml/min/1.73m^2)
This could be on basis of kidney biopsy OR
Active urinary sediment
OR pulmonary involvement (with diffuse alveolar hemorrhage)
Notable exclusion criteria were:
antiGBM disease
Use of PLEX, rituximab, cyclophosphamide or dialysis in the few days to weeks prior
Interventions
Everyone got induction with either cyclophosphamide or rituximab (per centre/treating physician choice) and steroids. The steroid induction was IV methylprednisone, at a dose of 1-3g, as per local protocols.
PLEX
Plasma exchange was ordered as 7 sessions in the first 14 days. Replacement was with albumin at 60 ml/kg, with plasma being allowed for replacement in someone at high risk of bleeding. The no PLEX arm got...no plasma exchange.
Steroid
This was a bit trickier to understand
All got IV steroids at start as mentioned above
Week 1: both groups got same dose
After this, the reduced dose group were approximately on 50% of the dose for the next few weeks, though the gap narrowed by 22 weeks, as can be seen in the table below
Week 22 - 52: both groups were on 5 mg prednisone, after which it was in hands of local investigators.
Table 1 lightly modified from Walsh et al, Trials 2013
Patients in whom refractory or early relapsing disease developed after randomization were treated with additional pulse glucocorticoids without plasma exchange.
After 3 to 6 months of cyclophosphamide treatment, patients received azathioprine to maintain remission until at least week 52, after which time the local investigators took over.
Outcomes
The primary outcome was a composite of ESKD or death, with ESKD being defined as 12 weeks of continuous dialysis or transplantation. There were some more secondary outcomes including sustained remissions, serious adverse effects, quality of life, and individual components of the primary outcome.
Analysis
For PLEX, as mentioned above, the hypothesis was that PLEX would be superior, with an HR of 0.64 (similar to the ESKD alone RR from their previous SR). From their calculation of event rates, they planned to randomize 700 patients to gather data on 164 primary outcome events, which would give them 80% power.
For the steroid arm, the hypothesis was of non-inferiority. The accepted non-inferiority margin was taken to be 11%. This meant that with 700 patients, they had 80% power to show that the incidence of ESKD or death was no more than 11 percentage points higher with the reduced-dose regimen than with the standard-dose regimen, at a one-sided alpha level of 0.05
Funding
An RCT of this size took a lot of funding from different organizations to pull through; National Institute for Health Research, United Kingdom; the Food and Drug Administration; the National Institutes of Health; the National Health and Medical Research Council, Australia; the Canadian Institutes of Health Research; the French Ministry of Health; the Research Committee on Intractable Vasculitides of the Ministry of Health, Labor, and Welfare of Japan; and the Japan Agency for Medical Research and Development (AMED): the Strategic Study Group to Establish the Evidence for Intractable Vasculitis Guideline.
In addition, Terumo BCT Mexico, Fresenius Medical Care Australia, Baxter Healthcare (Australia), and Asahi Kasei Medical provided in-kind plasma-exchange disposables.
Results
As planned, the investigators recruited 704 patients, all of whom were included in the intention to treat analysis. This required a herculean effort with 16 countries and 95 centres. About 660 patients were included in the per protocol analyses, as shown in the CONSORT figures below (from the supplement).
Table 1 shows the baseline characteristics of the enrolled patients. Some salient points worth noting:
About 40% were GPA (PR3+) versus the other 60% MPA
About 8-9% had prior history of AAV (so perhaps this was a relapse)
Median creatinine was 327 μmol/L (3.7 mg/dL) and just under a third had severe kidney disease with a creatinine > 500 μmol/L (5.7 mg/dL) or were already on dialysis
Only a quarter had pulmonary hemorrhage, with about 9% having severe pulomonary hemorrhage
Though almost all of them had kidney involvement, the proportion with kidney biopsy (versus just active urine sediment) is not mentioned
In terms of induction, cyclophosphamide remained the favorite, being the choice in a whopping 85% (mostly IV at 50% and oral at 35%) with rituximab for the remaining 15%
Table 1 from Walsh et al, NEJM, 2020
PLEX vs no PLEX
So how did they do? For the primary outcome (ESKD or death) PLEX didn’t do any better than not having PLEX. Whichever way you slice it (ITT analysis, adjusted, per protocol) there was nothing there (see figure 1 and table 2).
Figure 1A from Walsh et al, NEJM 2020
Table 2 from Walsh et al, NEJM 2020
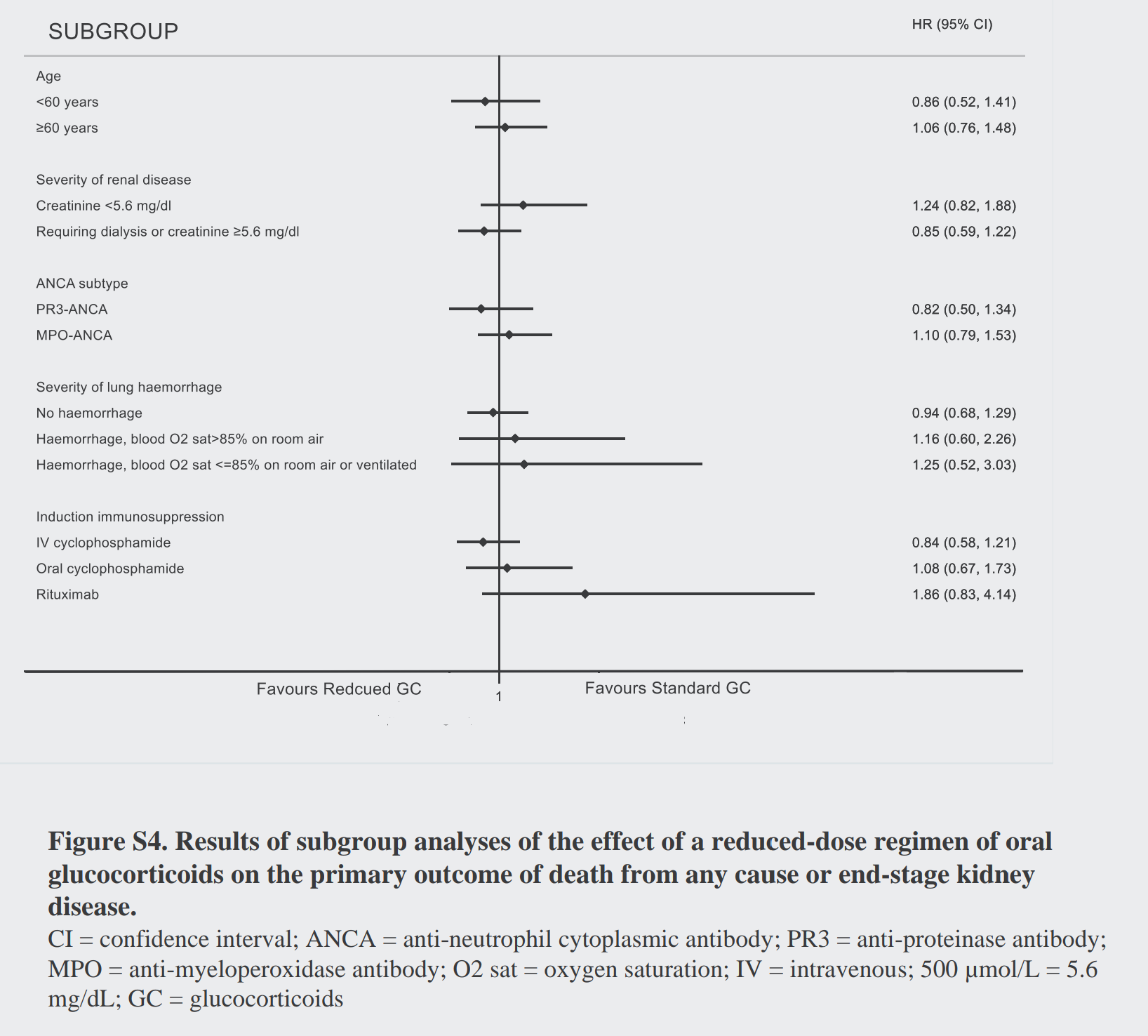
Maybe one of the subgroups did better? Though they don’t present the interaction p values, one would be hard pressed to see anything in the subgroups presented below (figure S3). Even for pulmonary hemorrhage, there was nothing (unless you want a Bayesian interpretation? Depending on your prior…).
Steroid outcomes
Noninferiority of a reduced-dose regimen of glucocorticoids to a standard-dose regimen in reducing death from any cause or ESRD
Reduced-dose group: occurred in 92 of 330 patients (27.9%)
Standard-dose group: occurred in 83 of 325 patients (25.5%)
Absolute risk difference, 2.3 percentage points (90% CI, −3.4 to 8.0), which met the criterion for noninferiority which was 11%. See figure below, and similar results as PLEX for the subgroup analyses.
Figure 1B from Walsh et al, NEJM 2020
The subgroup analyses for the steroids also are quite similar. Do you think there is anything going on with rituximab, like the NEJM editorialists do?
Secondary Outcomes
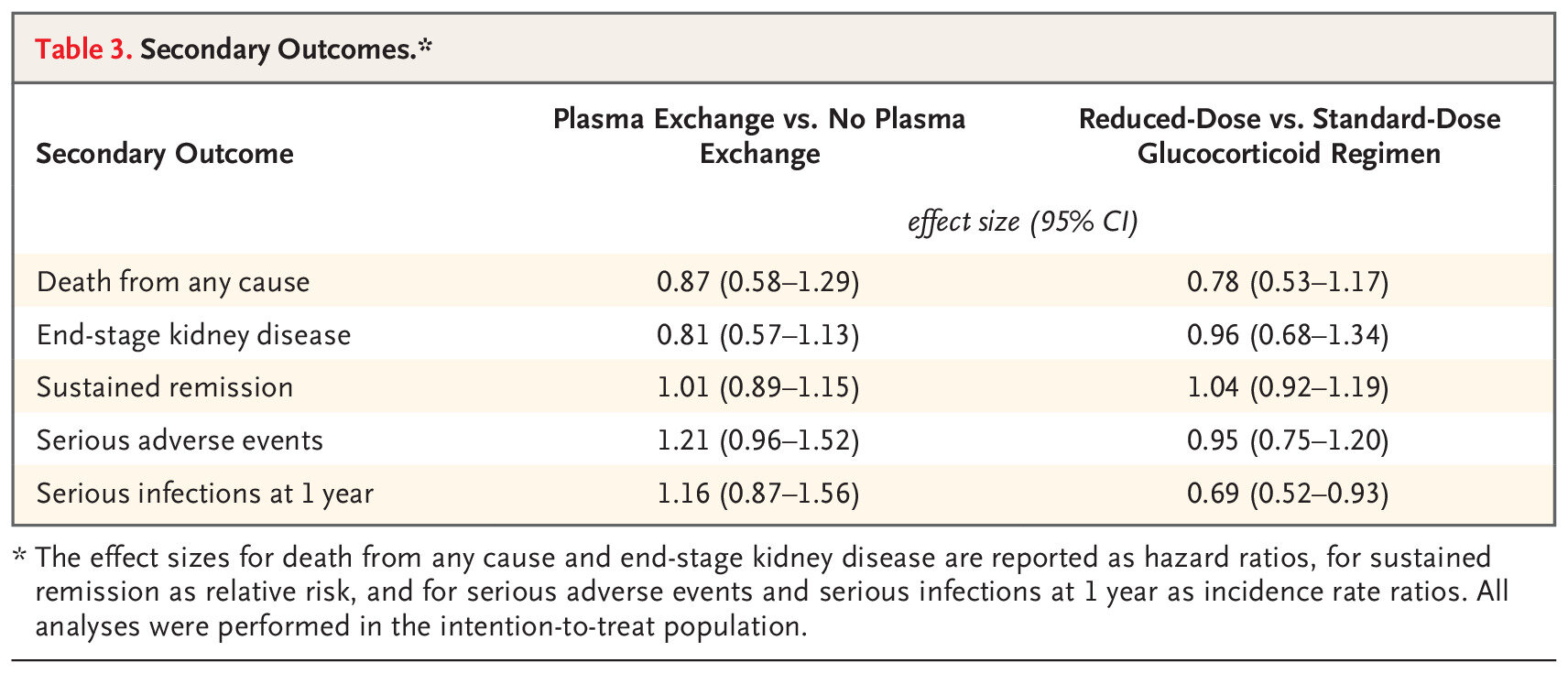
Similarly, nothing in terms of differences for the secondary outcomes: sustained remission, ESKD, death (separately considered) or the safety outcomes. A notable exception was that reduced dose steroids reduced the risk of serious infections. The quality of life findings are buried in the supplement, and didn’t show any difference either. Do you think there could be something in the ESKD alone outcome (RR 0.81)? Lets revisit that point later.
Table 3 from Walsh et al NEJM 2020
Discussion
Is this the end of the era of PLEX, and the beginning of the era of safely using lower dose of steroids in AAV? The main outcomes are pretty clear for mortality/ESKD reduction. Moreover, the win for reduced dose steroids is also quite clear, being clearly non-inferior as well as with less risk of severe infections.
But are we ready to accept the lack of benefit with no PLEX? There remains a reluctance to let go of PLEX with a few narratives quickly arising:
The lack of a need for kidney biopsy. This was a decision probably taken on pragmatic reasons: sometimes the patient is so sick (for example, in the intensive care unit with known ANCA and pulmonary hemorrhage), we don’t have time to wait for a biopsy. Patients without a biopsy may indeed have milder disease - but very well may have had more severe disease. So it is unclear how big a deal this is. Some more analyses of patients who did and didn't have a biopsy would be interesting, nevertheless.
The 191 patients who had pulmonary hemorrhage makes this subgroup larger than the entire MEPEX trial
Could there be a signal in the pulmonary hemorrhage subgroup? Here is where people might be most reluctant to let go of PLEX. Could the HR of about 0.64 mean there is a benefit in patients with pulmonary hemorrhage, but the trial was not powered to show this? This was a 700 patient RCT, and the 191 patients who had pulmonary hemorrhage makes this subgroup by itself larger than the entire previous MEPEX trial!
ESKD benefit? Even in MEPEX, and the last SR, the benefit for ESKD was more pronounced than the benefit for ESKD/death. In the present trial, the point estimate for ESKD with PLEX is 0.81 - and a quickly performed Cochrane SR including PEXIVAS results reports that ‘plasma exchange as adjunctive therapy may reduce the need for dialysis at three (2 studies: RR 0.43, 95% CI 0.23 to 0.78; I2 = 0%) and 12 months (6 studies: RR 0.45, 95% CI 0.29 to 0.72; I2 = 0%) (low certainty evidence).’
Strengths
It was a large trial involving patients with ANCA-associated vasculitis, and we used an objective, easily ascertained patient-focused outcome. Adherence to the assigned treatments was good and there were few losses to follow-up.
Limitations
It was an open-label trial, which exposed it to potential bias resulting from crossover, differential use of other therapies, and differential outcome ascertainment. However, crossover was infrequent, no other therapies are known to reduce the risk of death or ESKD, and the outcome ascertainment was unlikely to be biased, need for dialysis and death being fairly objective outcomes. Second, although the trial was large for a study of a rare disease, some estimates had wide confidence intervals, and potential differences in outcomes with respect to plasma exchange or the reduced-dose glucocorticoid regimen remain possible, as discussed above.
Conclusion
So is this curtains for plasmapheresis in ANCA vasculitis? Or do you remain a believer, like the NEJM editorialists - or perhaps you would like to do it for pulmonary hemorrhage, or to prevent ESKD despite the lack of benefit on mortality? Needless to say, the reduced dose steroids is a clear win. Join us this week on NephJC to discuss your opinions and perspectives.
Summary by Verner Venegas, Nephrology Fellow, Mexico
NSMC Faculty