#NephJC Chat
Tuesday November 12 at 9 pm EDT
Wednesday November 13 at 9 pm GMT
Wednesday November 13 at 9 pm IST
Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N Engl J Med. 2019;
A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus.
Steven Fishbane, M.D., Aamir Jamal, M.D., Catherine Munera, Ph.D., Warren Wen, Ph.D., and Frédérique Menzaghi, Ph.D. for the KALM-1 Trial Investigators.
PMID: 31702883 Full Text at NEJM
Introduction
Uremic pruritus is a big concern among patients, but less so among doctors and medical directors. A DOPPS publication found decreasing prevalence of the most severe cases of pruritus but widespread under appreciation of the problem by medical directors.
“Overall, 65% of medical directors estimated that < 5% of their patients suffered from severe pruritus. Medical directors underestimated the prevalence of pruritus in 69% of facilities.”
Itching has been identified as an outcome worth studying by patients and other stakeholders in the SONG-HD initiative. See the patient voice features from the previous NephJC discussion for more.
Itching has been linked to poor sleep, decreased quality of life, depression and poor outcomes on dialysis.
No drug is currently approved for the treatment of uremic pruritus in the US but many options have been tried. AJKD published a meta-analysis that was covered in NephJC about uremic pruritus. One of the main takeaways from the review was the low quality of the evidence. The most commonly tested treatments were gabapentin and pregabalin which were found to be better than placebo at reducing itching.
In 2017, Mathur et al. ran a randomized controlled trial of nalbuphine for uremic pruritus. Nalbuphine is a μ-opioid antagonist and κ-opioid agonist. This 370 person RCT showed the high-dose to be superior to placebo. Note the significant placebo effect.
This week’s NephJC looks at difelikefalin for uremic pruritus. Difelikefalin is a small peptide molecule. It is hydrophilic and limited from crossing the blood brain barrier. It is a kappa receptor agonist. Opioid kappa agonists are anti-itch (while mu-receptors promote itch), have analgesic activity, but while mu-activity causes euphoria, kappa can cause dysphoria. Since difelikefalin is excluded from the central nervous system, the developers hoped to avoid addiction and dysphoria while maintaining the anti-itch activity. Phase 2 trials were encoraging but seem to have been presented only as posters.
The Study
Methods
Kalm-1 is:
multicenter, 56 US sites
randomized
placebo controlled
double-blind
The patients were hemodialysis patients with at least 3 months of treatment. Study drug was given as an intravenous bolus via the venous line at the conclusion of each dialysis treatment for 12 weeks. The dose of difelikefalin was 0.5 mcg/kg. Phase II studies had looked at various doses and did not find a convincing dose response relationship above 0.5 mcg/kg.
To be enrolled, all participants had to have an average Worst Itching Numerical Rating Scales (WI-NRS) score above 4 for a week. The scale goes from 0 to 10, with ten having the highest itch burden. A WI-NRS above 4 indicates moderate to severe itch. This is how the scale looks like:
Worst Itching Numerical Rating Scale
Patients were treated for 12 weeks, during that time they completed weekly WI-NRS as well as the 5-D itch scale survey and the Skindex-10 multidimensional questionnaire periodically to give more insight into any change in pruritus.
After 12 weeks, the subjects stopped study drug and reported their symptoms with a tool called the Short Opioid Withdrawal Scale to detect any symptoms of opioid withdrawal. The self-administered survey was supplemented with the Objective Opioid Withdrawal Scale administered by a trained observer.
Outcomes
The primary outcome was the fraction of patients who had a reduction in their WI-NRS of at least 3 points from baseline to 12 weeks.
The secondary outcomes included:
Change from baseline to week 12 in the 5-D itch scale total score
Change from baseline to week 12 in the Skindex-10 scale total score
Percentage of patients who had a decrease of at least 4 points from baseline to week 12 in their WI-NRS score.
Funding Source
The trial was funded by Cara Therapeutics, the maker of this drug. Three of the 5 authors are employees of the company. Additionally, it seems the manuscript was written by medical writers (named in the acknowledgements) and stated thus ‘All the authors participated in the interpretation of trial data. The manuscript was prepared by the authors, with medical writing assistance funded by the trial sponsor. The authors provided final approval for submission of the manuscript for publication.’ It is not clear if any of the academic authors conducted the analysis - since here it states they interpreted the data.
Results
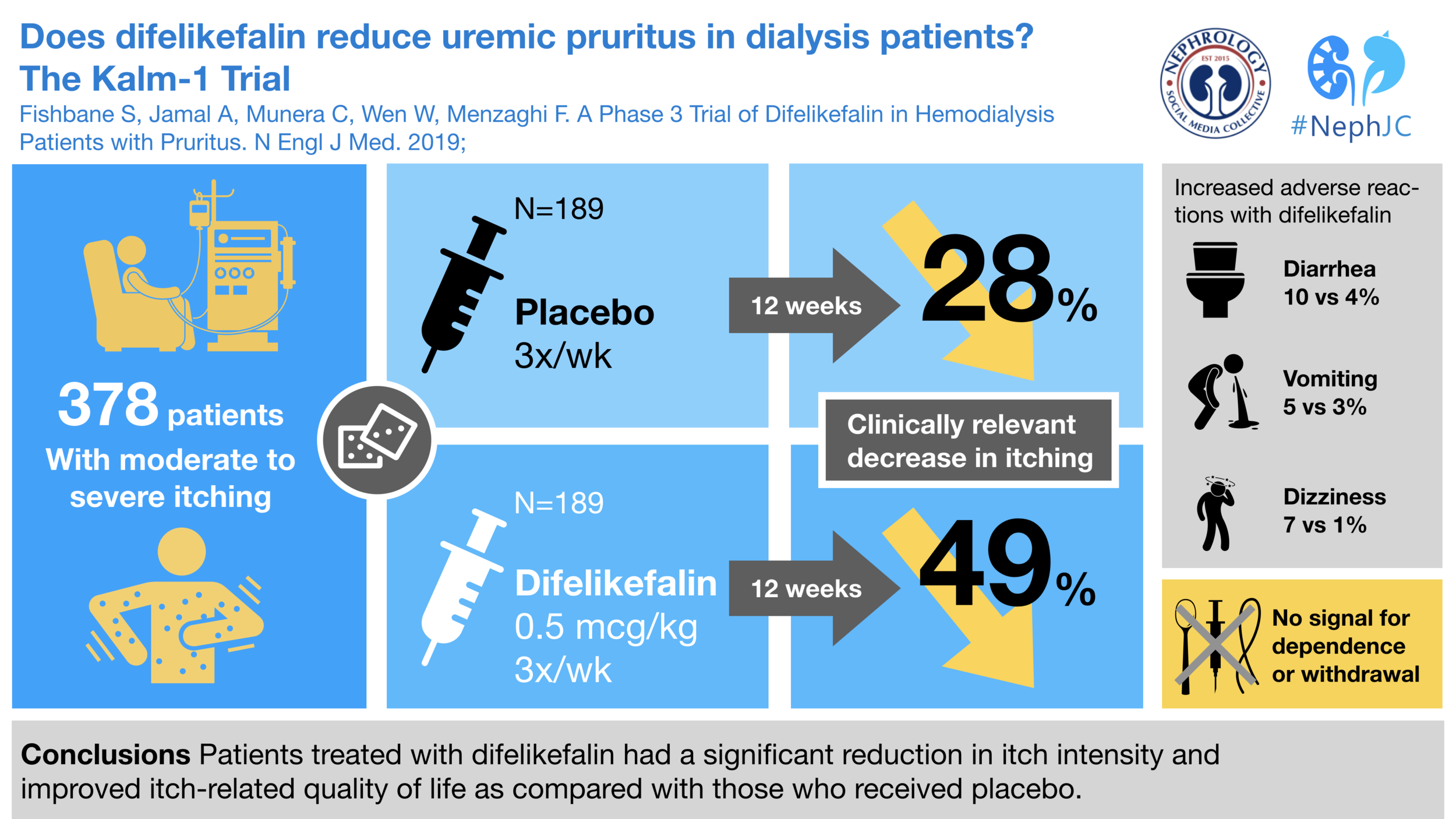
They enrolled and randomized 378 patients. You can see the demographics and baseline characteristics in Table 1. Dialysis vintage was about 5 years, and about 40% were black. The authors pointed to two particular numbers, the baseline WI-NRS and the baseline use of anti-pruritic medications. Though gabapentinoids had been identified in the previous SR as showing the most promise, none of the participants in this trial were on them - perhaps because it may have been off-label?
The traditional figure 1 has been banished from the main article to the supplement but since this is NephJC you can see figure S1 right here.
The primary outcome showed increased effectiveness for difelikefalin compared to placebo. Difelikefalin also bested placebo in all three of the secondary endpoints and this occurred regardless of baseline use of anti-pruritic medications..
The drug seemed to work fast and the lines appear to still be diverging at the end of 12 weeks. There is a 52-week extension for all of the patients involved of the study. It would be interesting to see the efficacy data over the long term.
Adverse Events
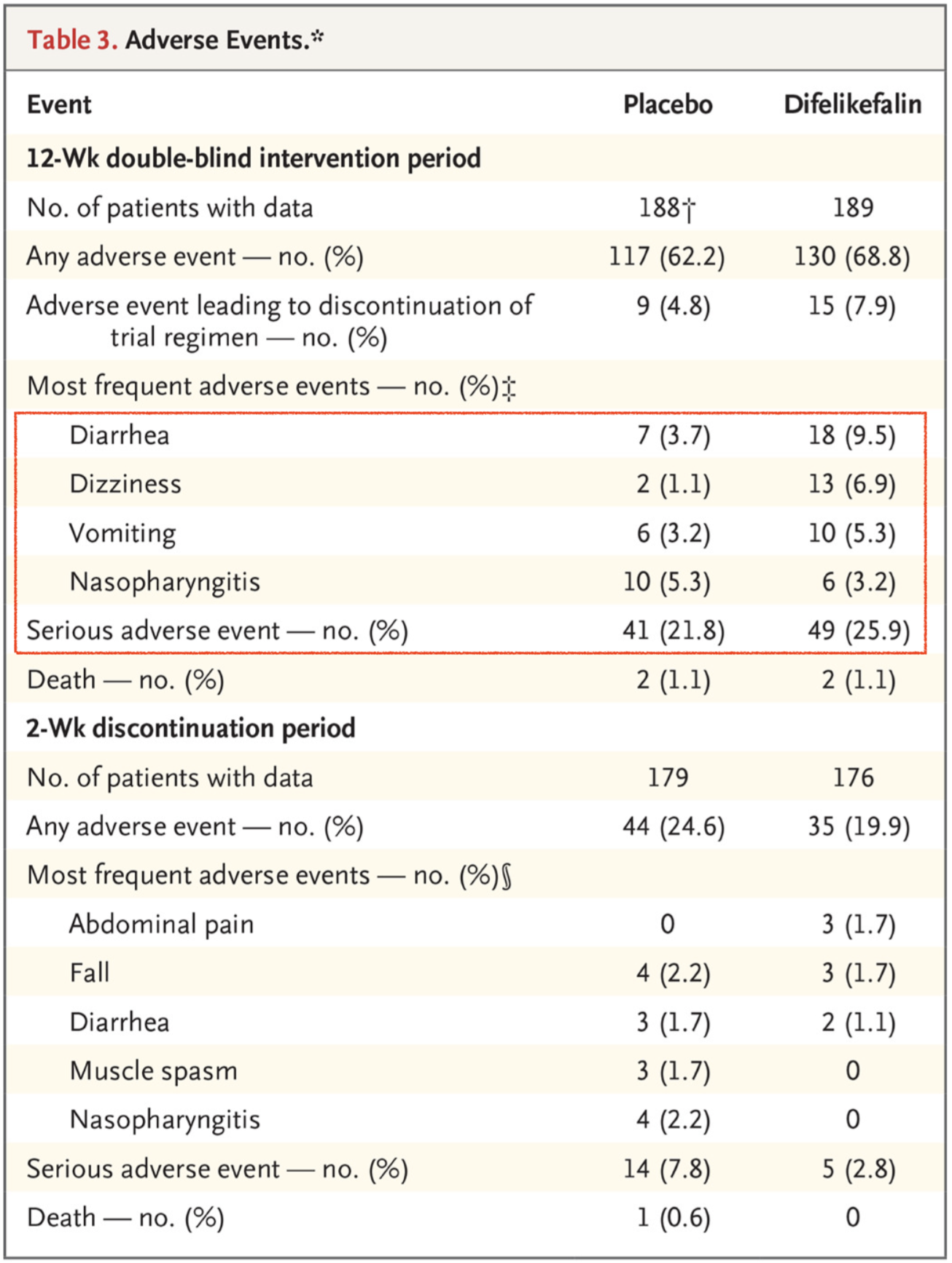
As typically seen in dialysis studies there was a very high rate of adverse events but they were generally balanced between groups with the exception of diarrhea, vomiting, and dizziness which were all increased with difelikefalin compared to placebo.
Serious adverse events seemed to be equally distributed between the two arms, 41 with placebo and 49 with difelikefalin.
There was no indication of any dependence or withdrawal. There was no dysphoria, hallucinations, or euphoria related to the differences between the groups.
Conclusion
Difelikefalin is a new type of drug in nephrology. It is a drug designed to ameliorate a symptom. It is supposed to make patients live better lives rather than just longer lives. However because it is treating a symptom we need to rely on new tools to see if the intervention is working. There is no creatinine, blood pressure, or other marker of surrogate outcome to track. All we can do is listen to our patients stories and see if they improve with this medication. Difelikefalin appears to do just what it claims to do, relieve itch symptoms.
Limitations
This is a short trial, of 12 weeks, and longer term data would be helpful to understand if the effect of the drug is persistent. AN open label continuation of the trial is ongoing which will answer this question.
The drug is given intravenously - and is dialysed. So dosing it post dialysis is useful and easy. However, patients on peritoneal dialysis also have itching. This came up on the Q/A - apparently an oral formulation is under development and may help answer this question.
Lastly, the patients were allowed to be on their baseline anti-pruritis medications, but none of them were on gabapentinoids. Should that be the next comparator to be studied in an RCT?
Summary by Joel Topf, MD FACP
Detroit, Michigan
Disclosure: as can be seen on page 4 of the supplementary Appendix, I was a site investigator on this study. I also advised Cara Therapeutics prior to the release of the KALM-1 results.