#NephJC Chat
Tuesday October 25 at 9 pm EDT
Wednesday October 26 at 9 pm IST
Nature Medicine. 2022 Oct;28(10):2124-2132. doi: 10.1038/s41591-022-02017-5.
Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus
Andreas Mackensen, Fabian Müller, Dimitrios Mougiakakos, Sebastian Böltz, Artur Wilhelm, Michael Aigner, Simon Völkl, David Simon, Arnd Kleyer, Luis Munoz, Sascha Kretschmann, Soraya Kharboutli, Regina Gary, Hannah Reimann, Wolf Rösler, Stefan Uderhardt, Holger Bang, Martin Herrmann, Arif Bülent Ekici, Christian Buettner, Katharina Maria Habenicht, Thomas H. Winkler, Gerhard Krönke, & Georg Schett
PMID: 36109639
Also check out this tweetorial by Matt Sparks
Introduction
Chimeric antigen T (CAR T) cells are actually living drugs. The way CAR T cell-based therapies work is by first isolating a patient's own leukocytes via leukapheresis and then sorting only the T cells (both CD4 and CD8 positive). The next step is to transfect the T cells with the necessary coding DNA to produce a new receptor capable of recognizing a target antigen (thus, no longer restricted to the native TCR-MHC paradigm). Moreover, one can also ensure that once binding to this antigen is achieved, the innate signaling can turn on potent machinery to destroy the cell that is recognized. Thus, CAR T cell-based therapies can produce a massive immune inflammatory response and requires inpatient admission to the hospital for close monitoring.
This technology was pioneered by Israeli immunologists Zelig Eshhar and Gideon Gross in 1989. (Gross et al Transplant Proc 1989)
Image from Totzeck et al, EHJ 2022.
The basic technology as described by Wikipedia:
Chimeric antigen receptor T cells (also known as CAR T cells) are T cells that have been genetically engineered to produce an artificial T cell receptor for use in immunotherapy.
Chimeric antigen receptors (CARs, also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors) are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric because they combine both antigen-binding and T cell activating functions into a single receptor.
The first commercially available CAR T cell therapy was Kymriah (tisagenlecleucel) which was FDA approved in 2017 for the treatment of acute lymphoblastic leukemia (ALL) in children (Styczyński, Acta Haematol Pol 2020). One sends frozen cells from the patient obtained after leukapheresis to the company to sort T cells and transfect with the CAR construct. Since then, CAR T cell-based therapeutics have revolutionized the toolbox for traditional chemotherapeutic resistant hematologic malignancies. Thus far, six CAR T cell-based therapies have been approved by the US FDA. One must remember that currently, CAR T cell therapy is a laborious process that includes many steps. You first need to isolate the patient cells, sort and transfect the T cells, condition the patient with chemotherapy, and deliver the therapy all while closely monitoring the patient in the hospital for signs and symptoms of cytokine release syndrome. Thus, CAR T cell-based therapy still has many hurdles to overcome. In development are off the shelf, fully transfected donor CAR T cells arriving fully transfected that can be immediately used. These still need to undergo rigorous testing before we see them used.
Table from NIH National Cancer Institute
Expectedly, interest in using CAR T cell-based therapies outside of hematologic malignancies has gained much attention particularly for autoimmune conditions, such as systemic lupus erythematosus (SLE). The track record for solid tumors has been rocky secondary to on target off tumor effects. For example HER2 directed CAR T cell-based therapy for HER2+ breast cancer led to significant cardiotoxicity because of HER2 expression in the heart.
SLE is a B cell mediated autoimmune disease. The mainstay of therapy has been prednisone with an anti-metabolite like mycophenolate mofetil (MMF) or alkylating agent like cyclophosphamide. Thus far, complete and durable depletion of B cells has not been achieved from the available treatment options.
Image from LandmarkNephrology. Note Aurora-1 is not featured, published in 2021.
The Authors hypothesized that depletion of B cells, using CAR T cells, will block auto-antibody formation and reverse glomerulonephritis and other end organ manifestations.
In proof of concept, two preclinical mice studies (Kansal, Sci.Transl.Med.2019, Jin, Cell Mol. Immunol. 2021) showed promising results. Hence, the authors conducted a first in-human case series of five patients with SLE, resistant to standard treatments.
The authors first described the use of CAR T cells in a single patient via a letter published in the NEJM in 2021. The patient was a 20-year-old woman with lupus nephritis class IIIA, nephrotic syndrome, and pericarditis who had failed therapy with steroids, cyclophosphamide, mycophenolate, tacrolimus, and B cell depleting therapies. After stopping all prior therapies, she underwent lymphodepletion and infusion of CD19 CAR T cells. Subsequently, she achieved serological improvement with rapidly decreasing anti-dsDNA levels and normalization of complement levels. Clinically, her proteinuria and lupus activity scores all decreased significantly.
Currently, patients with treatment refractory active SLE do not have curative treatment options. CAR T cell treatment in a single patient above led to dramatic improvement in lupus nephritis. As next step, researchers aimed to study CAR T’s tolerability and efficacy over a larger sample size.
The Study
It is a single center study conducted in Germany by the Department of Internal Medicine (Rheumatology and Immunology) of the Friedrich Alexander University Erlangen-Nurnberg under a compassionate use program.
Terminology
Design
Small case series of 5 patients. Intervention with CD19 directed Chimeric Antigen Receptor T cell receptor.
Study Population
Inclusion:
Diagnosis of SLE according to the EULAR/ACR 2019 criteria
Signs of active organ involvement, including kidney involvement (WHO III of IV)
Failure to respond to multiple immunomodulatory therapies including
Repeated pulsed glucocorticoids, hydroxychloroquine
Cyclophosphamide
Belimumab
MMF
Rituximab
An understanding of the procedure of CAR T cell therapy.
Exclusion (note, not clearly stated in the manuscript).
Presence of other autoimmune conditions is likely an exclusion criteria, although it was not clearly stated. For example, one patient was excluded due to concomitant psoriatic disease.
It is not clear if lupus cerebritis was used as an exclusion criteria. None of the five patients had CNS lupus.
What was the age criteria for inclusion or exclusion? It is not clearly stated but all subjects in the study were from between the ages of 18-24 years.
Sample Size:
Study did not prespecified sample size for accrual likely because it was used under a compassionate use program as a “proof of concept” study.
Over a period of one year of enrollment, five subjects were accrued in the study.
All patients had active SLE as documented by the SLEDAI-2K score.
SLEDAI-2K score stands for Systemic Lupus Erythematosus Disease Activity Index-2K (Gladman, D. et al. J Rheumatol 2002)
It is a scoring system of global disease activity. It has 24 items covering 9 organ systems. (SLEDAI-2K calculator https://www.mdcalc.com/)
Note that the SLEDAI-2K does not record improving or worsening, and does not include severity within an organ system.
CAR T cell Constructs
The structure of chimeric antigen receptor on a T cell generally consists of following parts:
Binding domain
Hinge region
Transmembrane region
Signaling domain
T cell activation domain
Examples of CAR construct. Image from Liu et al, Front Immunol 2019
How were the CAR T cells constructed?
Leukapheresis
T cell isolation (CD4 and CD8)
Lentiviral vector transduction of CAR vecture (as above) into autologous T cells
In vitro cell expansion of CAR T cells
CAR T administration logistics:
Figure 1a | Description of process and CAR T cell generation. Timelines of CD19 CAR T cell treatment in patients with SLE. Image from Image from Mackensen et al, Nature Medicine 2022.
Note that Day 0 in this study, is the day of CAR T cell product infusion.
It was a single infusion of a fixed dose of 1×106 CAR T cells per kg body weight
Prior to administering CAR T,
Immunosuppressive therapy was held
MMF and cyclophosphamide stopped 3 weeks before leukapheresis
Reduction of prednisolone dose to less than 10 mg per day.
Conditioning (chemotherapy) therapy to deplete the body's own T cells was given in the hospital from day -5 to -3.
Depletion of the body's own T cells helps CAR T cells to proliferate.
Lymphodepletion was done using two chemotherapy agents
Fludarabine 25 mg/m2/d intravenously (i.v.) from day −5 to day −3
Cyclophosphamide 1,000 mg/m2/d i.v. on day −3
Post CAR T administration, inpatient observation for toxicity was required for monitoring of the immediate post CAR T cell toxicities including
Cytokine release syndrome (CRS) (Wei. Sig Transduct Target Ther 2020)
Immune effector cell associated neurotoxicity syndrome (ICANS) (Holtzman. Neuro Oncol 2021)
In total, the patients were expected to stay in the hospital for a total of two and a half weeks (day -5 to day +10).
Endpoints
Primary and secondary outcomes were not specifically outlined. This small sample-size, descriptive study aimed to find tolerability and efficacy of CAR T cells and used various measures as detailed in the results section.
Funding
The study was supported by the following under compassionate use program:
Deutsche Forschungsgemeinschaft (FOR2886, CRC1181 and TRR221),
The Bundesministerium für Bildung und Forschung (BMBF; MASCARA),
The European Union (ERC Synergy grant 4D Nanoscope)
ERC Consolidator grant INSPIRE) and the
IMI-funded project RTCure.
Results
The study was conducted at a single site in Germany, between February 2021 to February 2022. Five adult patients were admitted to the hospital, with a diagnosis of SLE involving organ(s) refractory to multiple immunosuppressive treatment modalities, to receive autologous CD19 directed T cells. Out of 14 screened patients, seven met exclusion criteria (2 due to lack of organ involvement, 4 due to lack of other treatments received, 1 due to lack of understanding the procedure). Of the seven, 1 was refused treatment due to concomitant psoriasis. One patient did not consent. At the end, five patients received the therapy.
Extended Data Fig. 1 | Consort Flow Diagram. Image from Mackensen et al, Nature Medicine 2022.
Baseline Characteristics
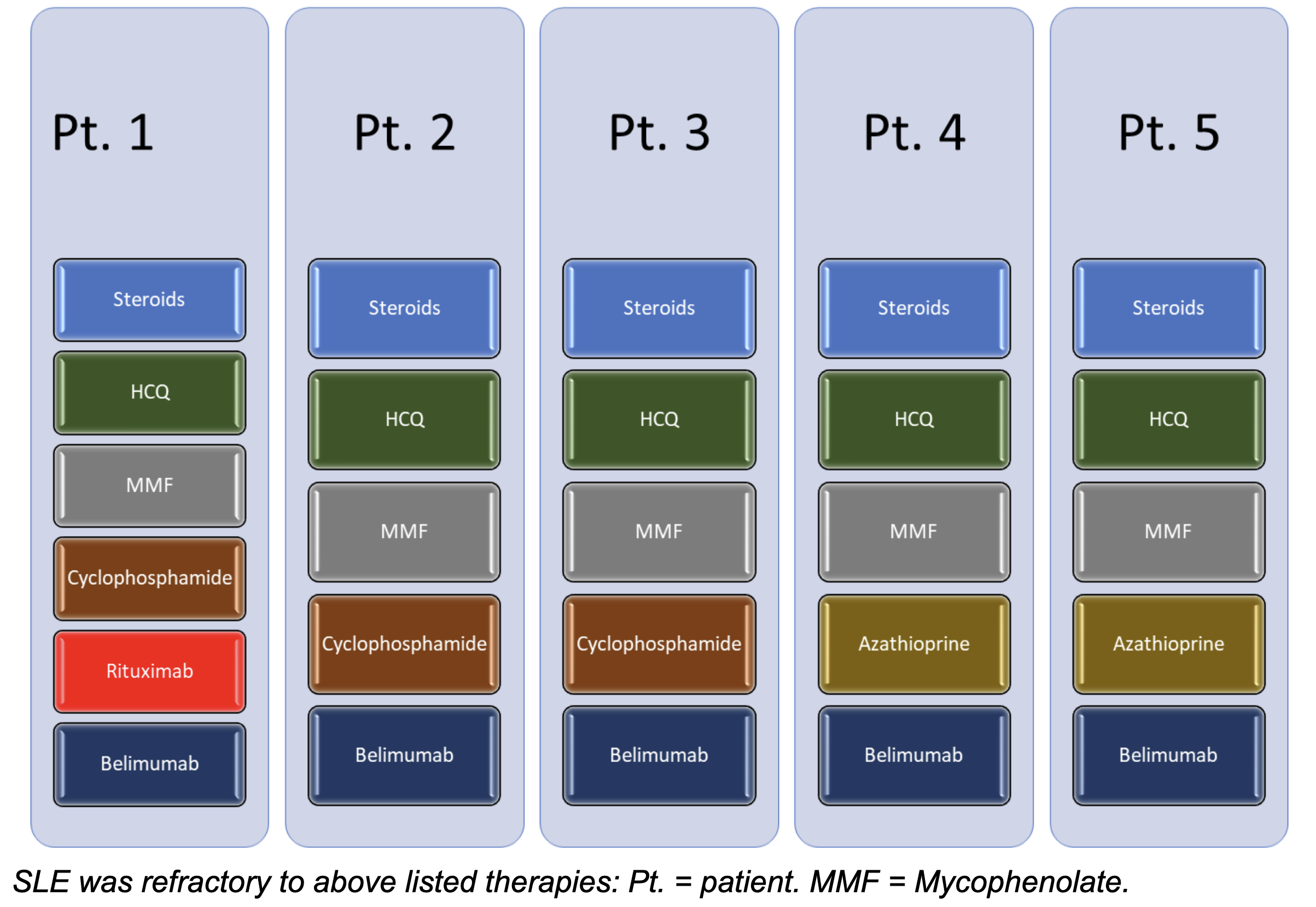
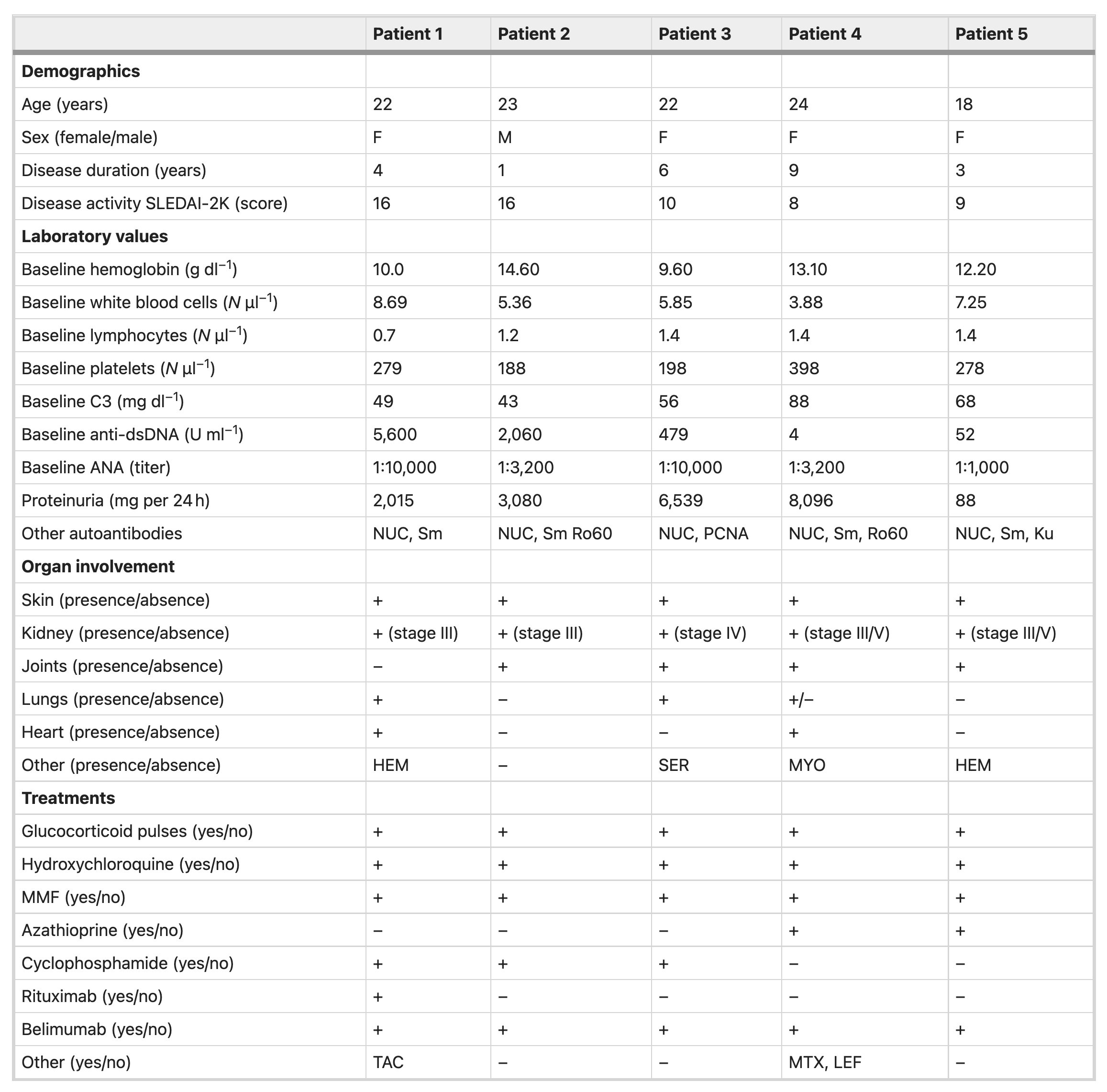
Of the five patients enrolled in the study, four were female and one was male, with age range from 18-24 years. Note that all patients had major organ involvement, including kidney (5/5), joints (3/5), lungs (2/5), skin (5/5), heart (2/5), hematologic (2/5). All patients were refractory to the following four immunosuppressive medications
Glucocorticoids
Hydroxychloroquine (HCQ)
MMF
Belimumab
In addition, cyclophosphamide (3/5) and rituximab (1/5) was used in some of the patients. Interestingly, no creatinine values given in the baseline characteristics.
Patient SLEDAI-2K scores range from 8-16. See Table 1.
Table 1 = Baseline characteristics of study participants. Image from Mackensen et al, Nature Medicine 2022.
Endpoints
The follow-up timeframe ranged from 5 to 17 months for the five study participants. The table below shows a summary of study observations.
Despite initial rapid expansion of CAR T in vivo by day 9, circulating CAR T cells then had a rapid decline in all five patients (Figure 2b).
Figure 2b = Circulating CAR T cell numbers in the five patients with SLE within the first 30 days after treatment (N=5). Image from Mackensen et al, Nature Medicine 2022.
As expected, there was swift and robust B cell depletion (Figure 2c). Total white blood count (WBC) recovered over a thirty days period (Figure 2d) due to improvement in other white cells (neutrophils, monocytes, etc)
Circulating B cell numbers (Figure 2c) and circulating total white blood cells (Figure 2d) in all 5 patients over 30 days. IImage from Mackensen et al, Nature Medicine 2022.
Clinical Efficacy
Study investigators noted a dramatic decrease in the SLEDAI-2K score in all five patients at three month follow up after CAR T cell administration (Figure 3a). Fatigue, a subjective and qualitative outcome measure, was also noted to have improved at three month follow up, among all five patients (Figure. 3e). Investigators noted at 3 months, resolution of proteinuria, improved complement level, and decreased anti-dsDNA level in all five patients (Figure 3 b-d). These results were maintained over a long term follow up (Figure 4b), but reconstitution of B cells was also evident (Figure 4a).
Figure 3a= SLEDAI-2K scores at baseline and 3 months after CAR T cell administration. Figure 3e= Fatigue measured by numerical rating scale (0–10) at baseline and 3 months after CAR T cell administration. Image from Mackensen et al, Nature Medicine 2022.
Figure 3 b-d = Lab indices at baseline and 3 months after CAR T administration (b=proteinuria, c=complement C3, d = anti-dsDNA). Image from Mackensen et al, Nature Medicine 2022.
Figure 4b= Long-term follow-up (FU) of the patients SLEDAI-2K score, complement, proteinuria, and anti-dsNDA. Bars indicate the respective follow-up periods. Image from Mackensen et al, Nature Medicine 2022.
Figure 4a = Time of recurrence of B cells after CAR T cell therapy. Image from Mackensen et al, Nature Medicine 2022.
B cells undergo class switching whereby an activated B cell changes its antibody production from IgM to either IgA, IgG, or IgE depending on the functional requirements. Researchers noted that after reconstitution of B cells, phenotyping revealed most B cells were naive B cells, and levels of memory B cells and plasmablasts were low to absent (Figure 4c). On a longer follow up, newly emerged B cells heavy chains were mostly IgM and IgD, instead of IgG and IgA likely due to lack of class switching of these cells (Figure 4d).
Figure 4c (partial) = Changes in the numbers of naïve B cells, memory B cells, plasmablasts and activated memory B cells between baseline (before CAR T cell therapy). Image from Mackensen et al, Nature Medicine 2022.
Figure 4d. Distribution of heavy chains in the BCRs at screening (‘original’ B cells at baseline, BL) and after CAR T cell treatment (‘reappearing’ B cells at follow-up) by mRNA sequencing of B cells. Image from Mackensen et al, Nature Medicine 2022.
Safety and Tolerability
Cytokine release syndrome (CRS)
Cytokine release syndrome or CRS is defined as a supraphysiologic response following any immunotherapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells. Symptoms can be progressive, must include fever at the onset, and may include hypotension, capillary leak (hypoxia) and end organ dysfunction.
CRS is a clinical diagnosis and manifested by fever (grade 1), hypoxia requiring supplemental oxygen (via nasal cannula = grade 2 or high flow oxygen = grade 3 or non-rebreather = grade 4), hypotension requiring vasopressor support (1 vasopressor = grade 3, more than 1 use of vasopressor = grade 4).
Study investigators noted elevation in inflammatory markers (Figure 5e, 5f) and CRS in 3/5 patients. Three patients had grade 1 CRS (fever). All three were treated with antipyretics. One patient additionally received tocilizumab (FDA approved therapy for CRS).
Fig 5 e, f= serum levels of C-reactive protein (e) and IL-6 (f) during the first 10 d after CAR T cell administration (all N = 5). Image from Mackensen et al, Nature Medicine 2022.
Discussion
The authors conclude that, in this case series of five patients with treatment refractory SLE with organ involvement, CD19 directed CAR T cell-based therapy was safe, tolerable, and effective in depleting autoimmune CD19 B cells. This led to improvement in symptoms, organ damage, and drug free remission.
Strengths
Provides feasibility for a study with a larger sample size
No commercial sponsor was involved
Early evidence that CAR T cell therapy offers highly effective therapy for SLE patients refractory to many treatment lines with a chance of drug free remission.
Study performed close monitoring of adverse events through inpatient observation. The therapy appeared quite safe.
Accrual of patients was monitored by an interdisciplinary specialist board monitoring
Robust short term safety data available
CRS appeared to be much lower than what is seen for malignancy based CAR T cell therapy studies. However, this is only a small study of 5 (likely ideal) patients, thus it will need to be replicated in larger studies.
Limitations
Limited sample size of five patient
Limited follow up: average 10 months; hence long term safety and efficacy are unknown
Efficacy in CNS lupus unknown
Study did not mention baseline creatinine or its improvement after CAR T cell administration
There was no biopsy before or after therapy to document histologic changes
1 patient had minimal change disease occurring after CAR T cell therapy.
Next steps?
Long term follow up to assess durability of treatment free remission after CAR T therapy.
Is it possible for outpatient administration of CAR T with close monitoring of patients so patients don't need to stay in the hospital for 2-3 weeks.
Randomized, double-blind, sham controlled trial
CAR T cell-based therapy could usher a new therapeutic option for patients with refractory disease. An exciting time indeed. However, several hurdles will need to be cleared before we see this used widespread. First, the safety of this therapy will need to be closely scrutinized in more patients and for longer duration. Second, how long will remission be achieved? This is a key question. If SLE comes back with a vengeance, what options will be available to these patients. Will it be amenable to standard therapy, or will CAR T cell therapy need to be used again. What will the infectious complications be in the long term with such robust loss of the entire B cell compartment. Lastly, the cost of this therapy is substantial and includes a close to two week stay in the hospital. This will limit the ability to use this therapy on a larger scale unless refinements are made to the protocol. Overall, this is an exciting development and something we will be all watching very closely.