Lancet. 2025 Aug 16;406(10504):705-718. doi: 10.1016/S0140-6736(25)01194-8.
Spironolactone in patients on chronic haemodialysis at high risk of adverse cardiovascular outcomes (ALCHEMIST): a multicentre, double-blind, randomised, placebo-controlled trial and updated meta-analysis
Patrick Rossignol, Faiez Zannad, Ziad Massy, Michel Azizi, Fatima Chorfa, Julien Coadic, João Pedro Ferreira, Francisca Saraiva, Dominique Mottier, Francis Guillemin, Willy Ngueyon Sime, Sanae Bouali, Bénédicte Rossignol, Joëlle Nortier, Isabelle Simon, Christophe Robino, Manuela Davin, Pierre M Bataille, François Chantrel, Nelly Castin, Vincent Esnault, Isabelle Kazes, Thierry Hannedouche, Nassim Kamar, Jean-Michel Achard, Caroline Fenerol, Carine Achard-Hottelart, Yves Dimitrov, Nicolas Girerd, Delphine Maucourt-Boulch, Luc Frimat; ALCHEMIST study group
PMID: 40818851
Why was this study done?
Check out the NephJC introduction for ACHIEVE and CTRL-C/V it here. Practically all the same reasons - except this trial was smaller than ACHIEVE (though still larger than any previous published trials) and performed in France (with Belgium/Monaco). One may think of alchemy as transmutating other metals into gold (which did not work) but the ancient alchemists also sought a panacea - a remedy for all diseases. In this case the ALCHEMIST (Aldosterone Antagonist Chronic Hemodialysis Interventional Survival Trial) was conducted to examine if spironolactone would be a panacea specifically for CV mortality in dialysis.
How was the trial done, and what did it show?
ALCHEMIST was a multicentre, double-blind, randomised, placebo-controlled, event driven trial conducted at 64 centres in France, Belgium and Monaco. Eligibility was adult patients with ESRD on HD (no PD patients unlike ACHIEVE) with at least one CV risk factor or comorbidity. Notable exclusions were recent unscheduled dialysis or hospitalization for hyperkalemia, or a serum K > 5.5 mmol/L. Similar to ACHIEVE there was a 4 week active drug run-in period with 25 mg spironolactone every other day and only those whose serum K remained < 5.5 were randomized. The intervention was the same dose of spironolactone, but titrated up to 25 mg daily, or 1:1 placebo. The primary outcome was a composite of CV death, non-fatal myocardial infarction, acute coronary syndrome, stroke, or HF hospitalization. Among the several secondary outcomes, notable were hyperkalemia (K > 6 mmol/L) and quality of life (KDQoL, SF 36 and the Minnesota living with HF questionnaire). They assumed an 18% event rate, and a 30% risk reduction, thus requiring 216 events in 750 participants over 2.5 years. Delays with COVID limiting recruitment, a lower than expected event rate (12% rather than 18%) and funding drying up meant the trial was concluded with 157 (rather than 216) events.
From the figure 1, of the 794 in the run-in period, 91 developed hyperkalemia (~ 11%) and 2 died during the run-in period. The mean age was ~ 70 years, ~ 70% had DM, and ~ 25% were on RASi. Notably, ~ 45% were on a potassium binder at baseline, and ~ 45% were on HDF, not just HD. At the time of trial termination (~ 33 months) about 38% participants had died. The primary outcome occurred in 78 (24%) of 320 patients in the spironolactone group and in 79 (24%) of 324 patients in the placebo group thus providing a perfect HR of 1.00 (95% CI 0.73 to 1.36; p=0.98). None of the other outcomes were also significantly different. Interestingly, hyperkalemia was only slightly (12%) higher with spironolactone, a difference which was not significant. The subgroup analysis was pretty consistent - with no interaction by sex. They did perform a quick and dirty meta-analysis (as Lancet sometimes asks the authors to do) but it is not worth wasting time or words on it since it does not include ACHIEVE and a separate and better meta-analysis was also conducted and published (see below).
Lancet . 2025 Aug 23;406(10505):811-820. doi: 10.1016/S0140-6736(25)01153-5. Epub 2025 Aug 18.
Safety and efficacy of steroidal mineralocorticoid receptor antagonists in patients with kidney failure requiring dialysis: a systematic review and meta-analysis of randomised controlled trials
Lonnie Pyne, Patrick Rossignol, Cameron Giles, Mats Junek, Patrick B Mark, Martin Gallagher, Janak R de Zoysa, P J Devereaux, Michael Walsh
PMID: 40840478
Why was this study done?
This was basically an update of their previous SR/MA (Quach et al, AJKD 2016) which did report the impressive 60% relative risk reduction with spironolactone in ESRD, but with the clear caveats that these results were at high risk of bias. Since ALCHEMISt (above) and ACHIEVE (NephJC summary) were now completed, this updated SR/MA became important to quantify the overall body of literature.
How was the study done, and what did it show?
This was an update of their previous systematic review, and uses the same methodology. In this case, the previous literature search had been up to 2015, and they have merely updated the search to 2025. The primary outcome for this meta-analysis was cardiovascular death, with other outcomes being secondary including hyperkalemia. The other notable thing, was a subgroup analysis by risk of bias, grouping studies that were at low risk of by separately from studies that were high risk of bias. This was the logical next step, given the high risk of bias noted with multiple trials in the 2016 reviewed. The rest of the methods are pretty plain vanilla as far as systematic reviews are concerned.
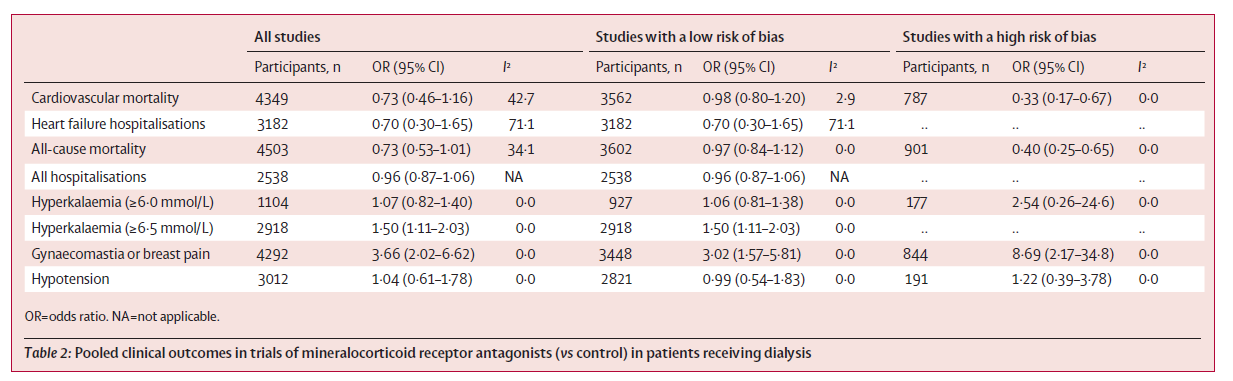
Overall, they just had 2 trials to be added to the previous ones, namely ACHIEVE and ALCHEMIST. Only one trial was that of eplerenone, whereas the others were spironolactone. From these 19 trials, 5 were considered low risk of bias, whereas the remaining were at high risk of bias, mostly because of unclear random sequence generation, allocation methods, absence of masking, and lost to follow-up being the issues. In their table 2, they report the overall results as well as by grouping based on the risk of bias, and the thing to appreciate is the higher heterogeneity in the overall analysis, with an I^2 ranging from 34-71 for the clinical outcomes. Once the grouping is changed, the heterogeneity comes down nicely, though it does not completely disappear.
Only restricting to the trials with low risk of bias there was no difference in cardiovascular death (pooled OR 0.98, 95% CI 0.80 to 1.20). Similarly, there was no significant difference in heart failure hospitalizations (OR 0.70, 95% CI 0.30 to 1.65), and all cause mortality (OR 0.97, 95% CI 0.84 to 1.12). The increase in hyperkalemia was only significant where it was defined as > 6.5, with a 50% increase (OR 1.50, 95 CI 1.11 to 2.03). As expected, there was an increased risk of gynecomastia, but no increase in the risk of hypotension.
What are the implications (ALCHEMIST and Meta-Analysis)?
Overall, the results are pretty consistent in demonstrating a lack of effect. Especially, if we restricted the studies at low risk of bias, the lack of benefit is extremely robust with little heterogeneity.
Interestingly, the ALCHEMIST trial did not show the sex based difference that was seen in ACHIEVE supporting our conclusion to mostly ignore that. The lower risk of hyperkalemia seen in ALCHEMIST is interesting, but it is important to keep in mind that roughly half the patients were on a potassium binding agent. These patients were also on hemodiafiltration, and there may be other idiosyncratic issues, such as the diet and practice patterns in that part of the world which may be different allowing for this. Lastly, the rates of hyperkalemia demonstrated in the run-in period was also quite similar, at about 10% to that seen in ACHIEVE.
Thus these 2 studies, paired with ACHIEVE should help prove that far from being up a panacea, spironolactone is merely fool's gold in the end-stage kidney. If you use it, use it for hypokalemia or hypertension, not for cardiovascular benefits.
Summary by Swapnil Hiremath
Reviewed by Cristina Popa