#NephJC Chat
Tuesday, January 14, 2019 at 9 pm Eastern
Wednesday, January 15 at 9 pm IST
Wednesday, January 15 at 9 pm GMT, and 1 pm Pacific
Transplantation. 2019 Oct;103(10):2012-2030. doi: 10.1097/TP.0000000000002762
Optimizing Mycophenolic Acid Exposure in Kidney Transplant Recipients: Time for Target Concentration Intervention.
Metz DK, Holford N, Kausman JY, Walker A, Cranswick N, Staatz CE, Barraclough KA, Ierino F
PMID: 31584924 Full Text at Transplantation
Nephrologists who care for patients post-transplant are aware of two things
We accept and understand the need for routine tacrolimus monitoring and
We neither accept nor understand mycophenolate mofetil (MMF) monitoring.
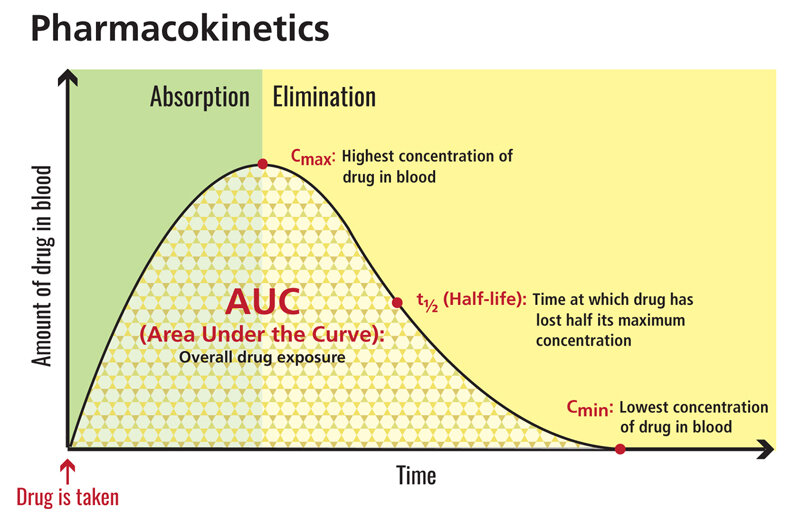
While literature reviews are usually not fair game for a journal club, this paper is important as it will provoke discussion about how we should be adjusting MMF doses in the post-transplant population. Trigger warning: this summary includes the terms pharmacodynamics and pharmacokinetics along with far too many acronyms. For a quick refresher, this graph summarizes the basic concepts:
Background
Immunosuppressant dosing aims for sufficient immunosuppression to prevent rejection while avoiding drug toxicity. This requires an understanding of inter-patient variability in pharmacokinetics (PK) and pharmacodynamics (PD). MMF was originally introduced as a fixed-dose (FD) drug but it displays wide variability in PK between patients, resulting in a 10-fold range in mycophenolic acid (MPA) exposure. Concentrations can vary between individuals depending on the extent of absorption and the rate of elimination. Dosing to a therapeutic range, known as therapeutic drug monitoring (TDM) and dosing to a target concentration, known as target concentration intervention (TCI) has the potential to maximise immunosuppression while limiting toxicity. TDM uses a range of concentrations between ineffectiveness and toxicity where TCI targets a specific concentration. Two randomised controlled trials (RCT) of concentration controlled dosing (CCD) in kidney transplantation showed substantially reduced kidney rejection when doses were individualized a target MPA area under the curve in the 12 hours post dose (AUC0-12) over a decade ago, but the benefit of CCD in comparison to FD remains contentious. MPA AUC0-12 is estimated either by PK profiling, necessitating multiple samples over 12 hours, or from a limited number of samples using a population PK model (MAPBE).
We have to quench our quest for the holy grail of measuring pharmacodynamic effects (immunosuppression level) with pharmacokinetic measures (drug levels). For tacrolimus a simple trough level seems to suffice; likewise, cyclosporine measurement started off as trough, before we realized that troughs don’t provide a good measure of drug exposure and peak, or C2 levels, became more commonly used . For MMF/MPA, things are trickier than this - and AUC seems necessary to make any sense of the data. These points are made very nicely in a couple of introductory figures from the authors, as seen below (legends ours, see paper for detailed explanation).
Figure 1 from Metz et al, Transplantation 2019
Drugs A, B and C may be dosed using fixed doses, though B requires a higher dose, and C a lower dose. The wide variation in drug D indicates that drug may benefit from pharmacokinetic monitoring.
Figure 2 from Metz et al, Transplantation 2019
Balancing toxicity and benefit with pharmacokinetic monitoring. Drugs are useful if benefits outweigh toxicity. Often this may be dose - or drug level dependent, and in the case above, at a level of 40, most of the benefit is seen, with little (though not negligible) risk of toxicity. Without knowing drug levels, one cannot weigh the tradeoffs.
Literature review
A systematic literature search was undertaken to identify studies in kidney transplant recipients which assessed:
The relationship between MPA exposure and benefit using MPA AUC0-12
The relationship between MPA exposure and toxicity (rejection, haematological toxicity and infection). Gastrointestinal toxicity was not examined as it is thought to be independent of plasma concentrations because it is due to direct gut mucosa toxicity from MPA.
The search was conducted in the usual databases with a properly done search plan. Though this was a systematic review, the data here is quite heterogeneous, and a qualitative synthesis is presented, rather than a quantitative meta-analysis.
The benefit of MMF CCD by RCT 6029 articles were identified initially and these were reduced by title, abstract and then full text review to 36 publications which were included in the systematic review.
Is MPA/MMF monitoring beneficial?
27 cohorts, totalling 3794 patients, were identified that assessed the relationship between MPA AUC0-12 and rejection (see table 1 for details). A statistically significant relationship between MPA AUC0-12 and rejection was evident in 20 of the 27 cohorts. This singular fact should make us all pause for a moment and ask if we measure MPA AUC0-12 in our centre.
The authors also reviewed 3 TCI trials which optimised MMF dose using MAPBE (see table 3 for details of the RCTs).
The first was a randomised concentration controlled trial (RCCT) which randomised 150 patients to 3 MPA AUC0-12 target groups; low, 16.1 mg/L.h; medium, 32.2 mg/L.h; and high, 60.6 mg/L.h. The primary end point, biopsy proven acute rejection, occurred more frequently in the lower target groups: 27.5%, 14.9% and 11.5%, p=0.043. Increasing MPA AUC0-12 was associated with a reduction in the probability of rejection.
The second RCT, APOMYGRE, randomised 137 patients to FD MMF (2g/d) or TCI to a target MPA AUC0-12 of 40mg/L.h and showed that TCI improved MPA exposure. Of acute rejection episodes in the first 3 months, 70% were associated with an MPA AUC0-12 of <30mg data-preserve-html-node="true"/L.h while the rest occured in those with MPA AUC0-12 between 30 and 45mg/L.h. TCI dosing in APOMYGRE cost <1% data-preserve-html-node="true" of annual hospital and treatment costs after a renal transplant, compared with the cost saving in preventing a single transplant failure of 8% of total annual costs.
The third trial, OPERA, randomised 247 patients, considered to be at low risk of rejection, to either FD MMF 2g/d or MMR optimisation with an empiric increased dose of 3g/d for 10 days after transplantation followed by TCI to a target MPA AUC0-12 of 40mg/L.h. The primary outcome, biopsy proven rejection at 3 months, was lower than expected. The optimisation arm did not tolerate therapy as well, with significantly more dose reductions for adverse events. Whether this was due to overly cautious clinicians or patients since dosage decisions were left to individual clinicians is not explored.
Finally, the authors considered 2 trials which examined MPA dose individualisation using TDM.
The fixed dose concentration controlled trial (FDCC) randomised 901 patients to either FD 2g/d or CCD with exposure within 30-60mg/L.h. This approach did not improve MPA exposure, perhaps due to inadequate early dose increments by clinicians, and thus this trial was inconclusive.
The second TDM trial, Opticept, was the only RCT of CCD using trough MPA concentrations. MMF dose optimisation was by TDM to achieve MPA trough concentrations of ≥1.3 or ≥1.9 μg/mL. Changes in dose was left up to clinicians who unfortunately made dose adjustments that were insufficient to attain the planned exposure.
Both of these trials involved substantial numbers of patients, along with significant time and resources but would have been improved by either clear protocols for dose adjustments or greater education and support for the treating clinicians.
Does MMF/MPA monitoring reduce toxicity?
For this objective, 22 cohorts identifying 3225 kidney transplant recipients were selected. Overall, the results were somewhat mixed:
9 studies reporting a significant association
2 showing a trend
11 which failed to show a relationship
Unlike with acute rejection, here there was a difference with cycloporine treated patients (2/11 cohorts showing a significant relationship) compared to tacrolimus cohorts (5/6 cohorts reporting a significant relationship). Additionally, measurement of unbound MPA AUC seemed to show an association with toxicity more than total MPA AUC (3 cohort studies).
All these bewildering results are summarized in Table 4:
Table 4 from Metz et al, Transplantation 2019
So what can we learn from this literature review?
This literature review shows that fixed dosing of MMF risks leaving a proportion of our patients underexposed to immunosuppression and at a greater risk of rejection. But TCI dosing of MMF is difficult with MPA AUC0-12 requiring multiple blood levels placing greater demands on patients’ time and hospital resources. Future studies are needed and those planning such trials should consider an implementation science approach to trial design to avoid inadequate dose increments confounding results. It is clear to me that TCI for MMF should be incorporated into routine post-transplant management. MPA/MMF monitoring makes a difference. The question is not of why, but how can we monitor?
Summary by Cathy Quinlan
Pediatric Nephrologist, Melbourne