#NephJC Chat
Tuesday, October 104h 2025, 9 pm Eastern on Bluesky
J Ren Nutr. 2025 Aug 26:S1051-2276(25)00185-2. doi: 10.1053/j.jrn.2025.08.003.
Effect of protein supplementation on health-related quality of life in individuals with advanced chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials
Yasmin Iman, Krista Rossum, Amanda Krueger, Favian Co, Makan Pourmasoumi, Ruth Ewhrudjakpor, Nicole Askin, Rebecca C Mollard, Clara Bohm
PMID: 40876553
Introduction
Food is life, and for many, a meal is a pleasurable social event, so restricted diets can be (literally) distasteful. For decades, dietary protein restriction has been recommended for patients with CKD to help ward off the onset of dialysis. The reduced elimination and resulting accumulation of uremic protein byproducts in CKD is thought to cause further damage to the kidney. High protein intake has been linked to glomerular hyperfiltration, glomerulosclerosis, tubulointerstitial, and progression of CKD (Friedman et al, Am J Kidney Dis 2004). Low protein diets (<0.8 g/kg/day), on the other hand, have been associated with reduced uremia production with improved renal hemodynamics (Koppe et al, Am J Kidney Dis 2019) and were popular in the pre-RAS inhibitor era.
However, trials examining clinical outcomes associated with protein restriction in patients with CKD don’t necessarily support any particular threshold of dietary protein intake. Meta-analyses in patients with CKD, both with and without diabetes, have demonstrated no difference in mortality, progression of CKD, or onset to dialysis with low protein versus normal protein diets (Hann et al, Cochrane Database Syst Review 2020). A decrease in the number of participants who reach kidney failure has been demonstrated with very low protein diets (0.3-0.4 g/kg/day) compared to low/normal protein diets. Notably, quality of life was not examined in these trials, and some have reported worsening nutritional status among patients with severely restricted protein diets (Hann et al, Cochrane Database Syst Review 2020). Indeed in the longer term follow up of the MDRD trial, the risk of death was almost twice as high with the low protein diet (Menon et al, AJKD 2009). Given the lack of evidence supporting dietary protein restriction, the KDIGO guidelines currently suggest maintaining a protein intake of 0.8 g/kg body weight per day in adults with CKD G3–G5 (level 2C recommendation – low certainty of evidence) (NephJC Summary).
Protein and ketoacid supplementation have also been evaluated in patients with CKD. In one study, very protein-restricted CKD patients (0.3 g/kg/day) who received ketoacid supplements had slowed eGFR decline and reduced need for dialysis compared to CKD patients consuming a standard low protein diet (0.6 g/kg/day) (Garneata et al, J Am Soc Nephrol 2016). Ketoacids are the building blocks for amino acids but don’t carry a nitrogen load, making this approach theoretically a logical (albeit expensive and one that requires adherence with a large bill burden) one for CKD patients to mitigate malnutrition concerns from protein restriction while avoiding uremic consequences.
Amino-acid and transamination of ketoacid analogues of amino acids in order to synthesize protein. (Koppe et al, Nutrients 2019)
Unfortunately, a very low protein diet plus ketoacid supplementation failed to show benefit in a more recent pragmatic trial (Bellizzi et al, Am J Clin Nutr 2022). And, in general, it may be questioned if we are really seeing an improvement in kidney function with these protein-restricted diets or if, possibly, we are just seeing a reduced serum creatinine level from reduced muscle mass (Kabasawa et al, J Ren Nurt 2025). The KDIGO guidelines add a practice point (i.e. lower than 2D) that for CKD patients at risk of kidney failure, a very low–protein diet (0.3–0.4 g/kg body weight/d) supplemented with essential amino acids or ketoacid analogs (up to 0.6 g/kg body weight/d) may be considered if done under close supervision (NephJC Summary).
What’s missing from all the CKD-protein data so far is an examination of quality of life. It’s worth asking – if a highly restrictive diet delays the need for dialysis, but those extra days without dialysis have poor quality due to malnourishment, the stress of a draconian diet, or lack of strength/function, is this really a positive outcome after all? Furthermore, can protein supplementation, rather than restriction, improve quality of life in patients with CKD without causing harm? Iman and colleagues aimed to answer at least part of this question in the current study – a meta-analysis of RCTs examining the impact of protein supplementation on health-related quality of life in individuals with advanced chronic kidney disease.
The Study
Methods
Inclusion criteria
Study design: Parallel-designed randomized controlled trials (RCTs)
Study population: Adults with CKD Stage G4-G5 or those on maintenance dialysis
Intervention: protein, amino acid, or keto-analogue of amino acid supplementation ≥ 5 grams per day via any route (but it seems the intraperitoneal route was not included)
Outcomes: change in health-related quality of life (HRQOL) was pre-specified as the primary outcome. Secondary outcomes included albumin, pre-albumin, BMI, mid-arm muscle circumference, and measures of physical function (grip strength, gait speed, sit-to-stand test)
Time: duration ranged from 2 to 36 months, and was 3 or more months for the majority of included RCTs
Exclusion criteria
Studies were excluded if they included high protein diet interventions without protein supplementation, animal studies, observational trials, quasi-randomized, cross-over, and cluster-randomized trials, and non-English publications.
Risk of bias assessment:
The Cochrane Collaboration Risk of Bias tool was used to assess the risk of bias in six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and “other”). If even one domain was determined to be high risk of bias, the overall rating for that trial was listed as high risk.
Statistical analysis:
The meta-analysis was performed using Review Manager (RevMan v5, The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). The mean change in each outcome over the study period was examined for the intervention vs control groups. Pooled continuous data was expressed as the mean difference (MD) or standardized mean difference (SMD), utilizing three or more studies that had the same outcome measures. Pooled MDs and SMDs and 95% confidence intervals (CIs) were calculated using a random-effects inverse variance model. Statistical heterogeneity was quantified using the I2 statistic and was considered unimportant for values < 40%. All tests of statistical inference reflect a 2-sided α of 0.05.
Funding Source: The authors report no sources of funding.
Results
Seventeen RCTs, (total 955 patients) were included in the systematic review, and 14 were included in at least one outcome-specific meta-analysis (Figure 1).
Figure 1. Study selection flowchart, from Iman et al, J Ren Nutr 2025)
Included studies were performed in 11 different countries. Trial duration ranged between two and 36 months with most lasting 3-6 months. The majority of studies (14/17) included patients receiving dialysis. There were four types of protein supplementation in the trials: amino acid oral powder (5 studies) and/or infusion (3 studies), oral whey protein powders (4 studies), supplemental protein beverages (2 studies), and keto-analogues of amino acids (3 studies).
The characteristics of the seventeen included studies are summarized in Table 1.
Table 1. Individual Study Characteristics of Included Trials, from Iman et al, J Ren Nutr 2025
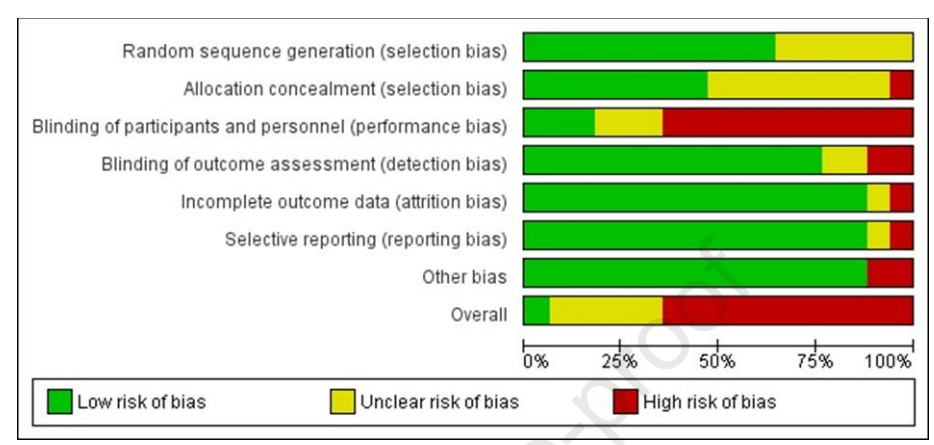
Only 1/17 trials were ranked as low risk of bias, 11 were ranked as high risk of bias (primarily due to lack of blinding), and 5 were listed as having unclear risk of bias due to lack of information.
Figure 2. Risk of bias assessment, from Iman et al, J Ren Nutr 2025
Outcomes
Primary Outcome
Health Related Quality of Life (HRQOL): 4 trials (n=318)
Only 4/17 trials examined HRQOL and only two of the four reported data needed for meta-analysis so no meta-analysis was performed for HRQOL. The four studies examining HRQOL included 244 HD patients and 74 PD patients. Qualitative analysis showed no significant difference in SF-36 and SF-12 scores between participants receiving protein supplementation versus standard of care, indicating no change in HRQOL.
Secondary Outcomes
Albumin: 14 trials (n=787)
Protein supplementation was associated with an increase in serum albumin compared with controls (MD = 1.76 g/L, 95% CI [0.67, 2.84], P<0.002, I2 = 88%) (Figure 3).
Figure 3a. Meta-analysis of studies examining the effect of protein supplementation on mean change in serum albumin levels (g/L), from Iman et al, J Ren Nutr 2025
Pre-Albumin: 3 trials (n=175)
There was no change in serum pre-albumin with protein supplementation as compared with controls (MD = 0.02 g/dL, 95% CI [-0.02, 0.05], P= 0.45, I2 = 56%).
Mid-arm muscle circumference: 4 trials (n=191)
There was no change in mid-arm muscle circumference between protein supplementation compared to control (MD = 0.38, 95% CI [0.00, 0.75], P = 0.05, I2 = 0%).
BMI: 7 trials (n= 311)
There was a statistically significant increase in BMI with protein supplementation compared to controls (MD = 0.29, 95% CI [0.10, 0.49], P = 0.004, I2 = 0%].
Figure 4b. Meta-analysis of studies examining the effect of protein supplementation on mean change in BMI (kg/m2), from Iman et al, J Ren Nutr 2025
Physical Function: 2 studies gain speed, sit-to-stand test (n=107); 2 studies handgrip (n=102)
This outcome did not meet pre-specified eligibility criteria for meta-analysis due to small number of studies and heterogeneity of physical outcome measures. In absence of meta-analysis, qualitative evaluation was performed showing no significant difference in physical function outcome measures between participants receiving supplements compared to the standard of care.
Discussion
This study was unable to achieve its primary aim, which was to shed some light on whether use of protein supplementation in patients with CKD may improve quality of life. There simply are not enough RCTs that have examined this question, and those that have have not done so in a standardized way to allow for meta-analysis. Qualitative analysis of existing trials, however, shows no impact on HRQOL, but given the variable nature of the trials (heterogeneous populations, interventions, and trial durations, to name a few), the only thing that can likely be firmly concluded is that we don’t have the type of evidence needed to make a recommendation on this.
The authors were able to conclude that protein supplementation was associated with a mean increase in serum albumin of 1.76 g/dL and 0.3 kg/m² in BMI, which is very similar to the findings of a previous meta-analysis on the topic, which found a mean increase in serum albumin of 1.58 g/dL and 0.4 kg/m² in BMI (Liu et al, PLoS One 2018). While the consistency in findings may be encouraging, it’s difficult to know exactly how to make this information actionable or if this has any clinically measurable impact at all. The heterogeneity of the included trials makes it impossible to know what supplement to recommend and who should receive recommendations to partake in such interventions. As discussed previously, the intricacies involved with protein intake in CKD make this a case where the devil really is in the details. Are we talking about implementing very low protein diets and then supplementing with ketoacids? Or a normal diet supplemented with ketoacids? Or a normal diet supplemented with whey protein? Or a low-protein diet supplemented with protein shakes?….you get the picture. When the nuances of implementation may have a clinically meaningful impact on patient outcomes, it's challenging to know how to utilize information to form a population-based recommendation. Furthermore, are these linked, in any way, to clinically significant outcomes? Is an increase of 0.3-0.4 in BMI likely to improve a patient’s life? If the increase in BMI takes a patient from underweight to normal weight vs normal weight to overweight, the outcomes could be different. Do we feel confident that increased albumin levels will improve health span for individuals with advanced CKD who ultimately transition to ESKD?
If nothing else, this trial brings to light the importance of changing the conversation on this topic. Moving away from a sole focus on surrogate markers and instead focusing on what we (and more importantly, patients) care about, HRQOL, is definitely a step in the right direction. Even the suggestion of supplementing with protein rather than restricting it in patients with advanced CKD allows us the opportunity to entertain ideas that challenge age-old dogma. Could it be that restricting protein (which does not delay or slow down progression but is often practiced, as it makes the creatinine number look better) actually leads patients to arrive to dialysis less strong and less healthy than if they were unrestricted? Would patients fare better both before and possibly after dialysis initiation if we increased protein intake? Needless to say - this is also not about less or more but about adequate protein intake. We do continue to think that very high protein intake (uncertain threshold, perhaps >1.5 -2 g /kg/day) is probably not good for the kidneys due to hyperfiltration injury. (Ko et al, J Am Soc Nephrol 2020).
The bottom line is that this meta-analysis seems to have come before the RCTs but hopefully can be used to guide future prospective trials examining this important issue.
Strengths of the study
Examination of HRQOL in studies including participants with advanced-stage CKD is of utmost importance, so it is a strength that this was named the primary outcome of this trial. Furthermore, there is a lot of uncertainty about what is best for patients when it comes to protein intake among patients with CKD, so efforts to build the evidence to guide these recommendations is highly relevant.
Limitations of the study
The major limitation is the lack of trials reporting on the primary outcome, HRQOL, so no meta-analysis was able to be performed. Other limitations include the heterogeneity of the intervention among trials (type of protein supplement, dose, duration) and short trial duration (3-6 months for most). Additionally, it wasn’t clear what level of dietary protein intake participants were adhering to while utilizing supplements in the trials, and adherence to the intervention was only reported in 2/17 studies. Finally, there was a high risk or unclear risk of bias in all but one trial.
Take-Home Message
The current evidence does not demonstrate improved HRQOL with protein supplementation in patients with advanced CKD. Protein supplementation is associated with a moderate increase in serum albumin and a small increase in BMI, but there is no consensus on what this means exactly (which supplement to use and what to do with protein in the diet while supplementing). Finally, it is not clear if an increase in serum albumin and BMI as a result of protein supplementation will ultimately lead to improvement in clinically meaningful outcomes.