#NephJC Chat
Tuesday June, 7th, 2022 at 9 pm Eastern (AEST June 8th, 11am)
Wednesday June 8th, 2022, at 9 pm Indian Standard Time and 4:30 pm BST (AEST June 9th, 2am)
JAMA. 2022 May 17;327(19): 1888-1898. doi: 10.1001/jama.2022.5368
Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial
Jicheng Lv, Muh Geot Wong, Michelle A Hladunewich, Vivekanand Jha, Lai Seong Hooi, Helen Monaghan, Minghui Zhao, Sean Barbour, Meg J Jardine, Heather N Reich, Daniel Cattran, Richard Glassock, Adeera Levin, David C Wheeler, Mark Woodward, Laurent Billot, Sandrine Stepien, Kris Rogers, Tak Mao Chan, Zhi-Hong Liu, David W Johnson, Alan Cass, John Feehally, Jürgen Floege, Giuseppe Remuzzi, Yangfeng Wu, Rajiv Agarwal, Hong Zhang, Vlado Perkovic; TESTING Study Group
PMID: 35579642
INTRODUCTION
The astute NephJC follower will remember the TESTING study (Jicheng Lv et al, JAMA 2017), and the wonderful summary for NephJC by Amit Langote. This 2017 multicenter, double-blind, randomized clinical trial compared oral methylprednisolone to placebo, examining the risk of disease progression in IgA Nephropathy (IgAN). It found that those treated with methylprednisolone were statistically less likely to progress to the primary composite outcome (40% decline in eGFR, kidney failure, or death due to kidney disease). However, it was terminated early due to 28 serious adverse events, including three infection related fatalities in the steroid arm.
Visual abstract created by @langoteamit for NephJC in 2017
Well we are back folks, TESTING steroids in IgAN again… again. We have a bigger cohort: 503 participants. We have the same composite outcome: 40% decline in eGFR, kidney failure or death due to kidney disease. And, we have a reduced dose methylprednisolone regimen.
As summarized by Diana Mina for Landmark Nephrology in 2017, steroid therapy in IgAN was first investigated in 1999 and has had varied results. Pozzi et al. found that when compared to IV methylprednisolone, control groups were more likely to demonstrate a 50% increase in plasma creatinine over 5 year follow-up (Pozzi et al, Lancet 1999). Conversely, Hector Madariaga reviewed STOP-IgA for NephJC in 2015, and this RCT demonstrated immunosuppression (including prednisolone) did not improve outcome in 177 individuals with IgAN (Rauen et al, NEJM 2015). There were however significant adverse events related to steroid therapy, including impaired glucose tolerance, more infection, malignant neoplasm, and one sepsis related death.
Timeline for steroid therapy in IgAN by Landmark Nephrology.
IgA nephropathy, apart from being the commonest primary glomerular disease, is also a heterogeneous entity. Some individuals will have some microscopic hematuria and nothing else. Others will have a rip-roaring crescentic IgAN with a relentless RPGN progression to kidney failure. And one can find everything in between: a bit of proteinuria, slow progression of CKD, nephrotic syndrome. How to decide whom to treat? Proteinuria, eGFR, age, blood pressure - all of these seem to matter, along with the pathology and intriguingly, ethnicity. Many ways to risk stratify have been developed - see the one of the most well-validated risk prediction studies that we discussed here (Barbour et al, JAMA IM 2019; NephJC summary by Tiffany Caza).
The lack of definitive data and reports of adverse events have led to the 2021 KDIGO guidelines suggesting consideration of a 6 month course of glucocorticoid therapy in those with IgAN at risk of progressive CKD despite maximal supportive care. KDIGO warns to proceed with extreme caution in those with eGFR <30ml/min/1.73 m2, diabetes, BMI > 30, latent infections, secondary disease, active peptic ulceration, uncontrolled psychiatric illness, or severe osteoporosis.
In summary, there is a signal that steroids likely have some benefit in a select higher risk population, but they also have more adverse effects. The TESTING 2.0 trial includes the original TESTING cohort we have discussed before, along with a reduced dose protocol (with added PJP prophylaxis) that was implemented following those original results. Let us examine if they could thread the needle this time.
THE STUDY
Design:
This was a randomized, double-blind, placebo controlled, multicenter clinical trial.
Run-in phase:
A run-in period of 12 weeks was used to optimize background therapy with ACEi/ARB (note, no SGLT2i here). Those that were adherent to treatment with persistent proteinuria (>1g/day) were then randomized centrally to receive either oral methylprednisolone or matching placebo. See more about run-in phases and what they can ACHIEVE in the NephJC summary by Manasi Bapat.
Randomization:
Randomization was conducted using a minimization algorithm based on four stratifiable variables (site, proteinuria, eGFR and endocapillary proliferation status on renal biopsy).
Intervention:
Study visits:
Monthly for 3 months, then every 3 months until month 12, then annually until the completion of study.
Study population:
Inclusion criteria
Biopsy proven diagnosis of primary IgA nephropathy AND
24-hour urine protein >1g/day AND
eGFR
20-120 ml/min/1.73m2 in full dose cohort
30-120 ml/min/1.73m2 in the reduced dose cohort
Exclusion criteria
Indication for immunosuppressive therapy, e.g.
Minimal change renal disease with IgA deposits
>50% crescents on biopsy
Contraindication to immunosuppression therapy, e.g.
Active infection
Malignancy
Current or planned pregnancy/breastfeeding
Systemic immunosuppressive therapy in previous year
Malignant/uncontrolled hypertension
Current unstable kidney function for other reasons e.g. macrohematuria-induced AKI
Age <14 years
Secondary IgAN
Those unlikely to comply with study protocol
Statistics:
Sample size
The trial required at least 160 primary outcome events to provide 90% power to detect a 40% overall reduction in hazard for methylprednisolone group which correlated to 500 participants.
The trial had initially been designed with a 90% power to detect 30% risk reduction requiring 1500 participants, assuming a baseline event rate of 7%. However the outcome was modified to 40% (rather than 50%) decrease in GFR, and data suggested event rate was 12%, and sample size was reduced to 750 - with an expectation of accruing 335 events over 5 years. With the suspension of the original TESTING, this was all revised yet again, taking into account feasibility after rebooting for the re-revised estimate for 160 events requiring 500 patients (a third of the originally planned sample with 335 events in 1500 participants).
Statistical analysis:
Multiple statistical models were applied
The effect of treatment on the primary and secondary endpoint was assessed using a Cox model with site as a random effect and baseline proteinuria, baseline eGFR and kidney biopsy finding the stratification variables.
Decline eGFR analyzed as the mean rate of decline and compared between groups with a t test
Adjusted annual event rates and absolute risk difference with confidence interval estimated using a poisson model adjusted by the stratification factors
Survival curves generated with Kaplan-Meier method
Outcomes:
Primary outcome:
Composite of
First occurrence of a sustained (>30 days) 40% decrease in eGFR OR
Kidney failure (requiring dialysis or kidney transplant)
OR
Death due to kidney disease
Secondary outcomes:
Sustained decreases in eGFR of 30%, 40%, 50% combined with kidney failure
Proteinuria reduction
eGFR slope
Adverse events
Safety assessment
As mentioned above, there were significant safety concerns including three fatalities and the study was halted in November 2015 (262 enrolled). A revised protocol with the methylprednisolone dose reduction (see above) was commenced from March 2017 with the recruitment of an additional 240 participants.
eFigure 1: Summary of sequence of events of the TESTING trial
Subgroups:
Planned subgroups were those associated with less favorable clinical characteristics. A single, unplanned subgroup was included related to methylprednisolone dose.
Funding
Funding was provided by various academic and research funds in Australia, China and Canada, in addition to Pfizer (previously known as the UpJohn company and the first pharmaceutical to synthesize and produce oral methylprednisolone). Funding agencies had no role in design, conduct, management, analysis, interpretation of data, preparation, review or approval of manuscript, and therefore unlikely to influence the outcome.
RESULTS
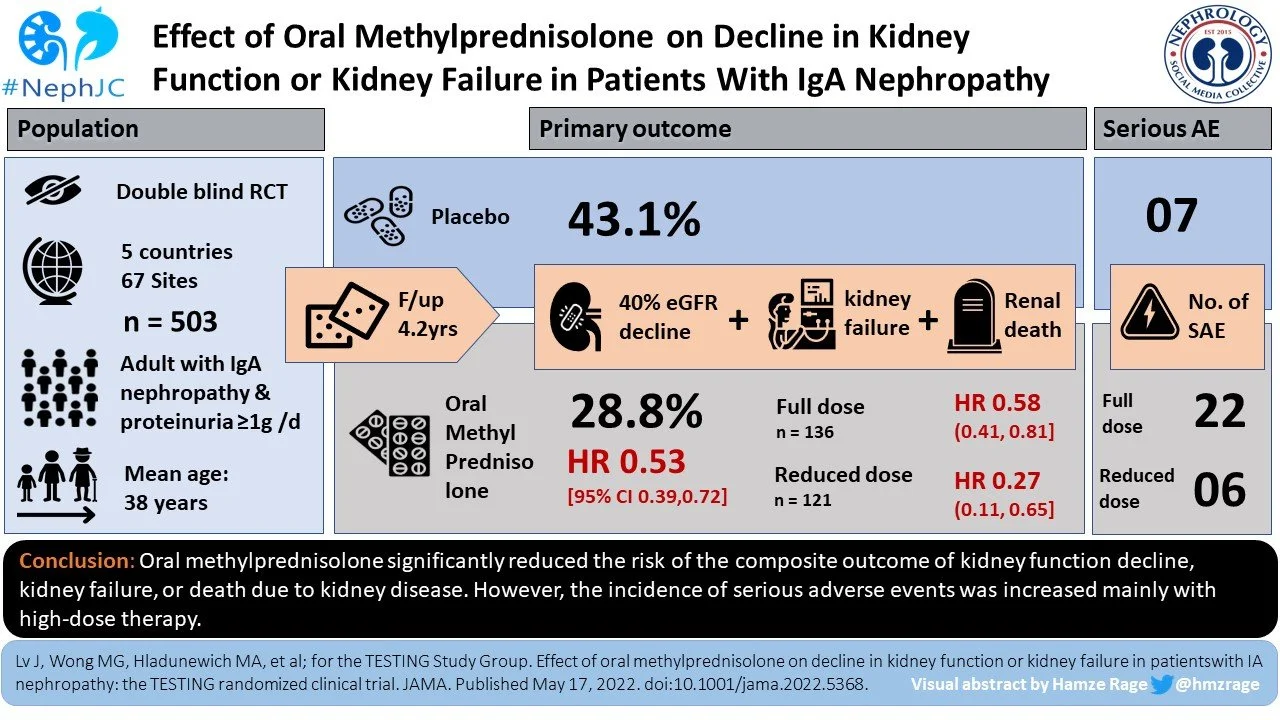
Between May 2012 and November 2019 a total of 950 eligible patients were screened at 67 sites around the world (5 countries: Australia, Canada, China, India, and Malaysia, compared to only China and Australia in original TESTING). Only 25 patients were of White/European ethnicity, with South Asian (63, ~ 12%) and Southeast Asian (33, ~ 7%) being slightly higher. Thus, the majority were of Chinese ethnicity (279, ~ 75%). Of these, 503 were randomized, 257 to the methylprednisolone group and 246 to the placebo group. Of the 503 included, 20 participants did not receive the assigned intervention, 52 discontinued prematurely (33 from methylprednisolone group) and 10 were lost to follow-up (3 who withdrew consent and 7 who lost contact) leaving a total of 421 completing the full treatment. All 503 participants were included in the primary and secondary outcome analysis.
Figure 1: Flow of participants
Baseline characteristics were consistent with epidemiologic data of IgAN (male predominance, lack of family history, prevalence of hypertension). Median age was about 36 years, median eGFR in the 55 - 60 ml/min/1.73m2 range, and median proteinuria about 2g/day. Blood pressure was well controlled, all but one participant was managed on an ACE inhibitor/ARB or both, with >80% on at least 50% maximum dose. Interestingly, despite it being a stratified variable, more participants in the methylprednisolone group had evidence of endocapillary hypercellularity (28% vs 22%). Compared to TESTING 1.0 which had 95% patients from China, the reduced dose cohort was ~ 50% outside China, and had higher GFR (in keeping with higher GFR needed for entry), with higher BMI, and less IFTA on biopsy.
Table 1: Baseline Characteristics of Study Participants (Lv et al, JAMA 2022)
Key outcomes:
The primary composite outcome - 40% eGFR reduction, kidney failure, or death due to kidney disease - occurred less frequently in those randomized to methylprednisolone group compared to placebo group (74 [28.8%] vs 106* [43.1%]; HR 0.53 [95th CI, 0.39 - 0.72]; p <0.001), over a mean follow-up of 4.2 years. The TESTING 1.0 cohort contributed 6.1 years of follow up and the bulk of events (151) compared to 2.5 years from the later reduced dose cohort with only 27 additional events.
(*106 events are reported in the papers abstract and Table 2, though confusingly in Figure 3 below you’ll notice the numbers reported are 84 + 20, for 104 outcomes instead, for which the reason for the discrepancy is unclear)
Similarly, when compared with placebo, treatment with methylprednisolone significantly reduced the risk of secondary outcomes including total and rate of eGFR reduction, kidney failure requiring dialysis or transplant, and protein excretion.
Table 2: Primary and Secondary Outcomes (Lv et al, JAMA 2022)
Figure 2A: Time from randomization to primary outcome in all patients (Lv et al, JAMA 2022)
The effect of methylprednisolone on protein excretion was not sustained over the course of the trial, with no significant difference at 3 years post commencement of methylprednisolone therapy. The annual rate of loss of eGFR was significantly greater in the placebo group (about 5 vs 2.5 ml/min/1.73m2). Interestingly, this effect on loss of function was negated when the measurements from the duration of therapy (1-6 months) were excluded.
eFigure 3: Proteinuria and eGFR reduction by randomized group over time
There was no significant difference for efficacy between dose regimens (full dose vs reduced dose) or any of the prespecified subgroups. The only exception was ancestry, with significant greater benefit of methylprednisolone therapy in non-Chinese participants compared to Chinese participants, though both groups did have benefit with steroids.
Figure 3. Primary outcome, sub-group analysis
Adverse events:
Serious adverse events (SAE) were significantly higher in the methylprednisolone group vs placebo group (37 vs 8 total events that occured in 28[10.9%] vs 7[2.8%] subjects). There was a reduction in those that developed kidney failure from 67 (placebo group) to 50 (methylprednisolone group). These were primarily related to hospitalization due to serious infection (including pneumocystis jirovecii pneumonia, sepsis, tuberculosis with bacterial infection), and/or gastrointestinal bleeding. They also included clinically evident fractures, osteonecrosis and new onset diabetes mellitus. Alarmingly, across the trial there were four fatalities, all of whom were randomized to the methylprednisolone arm (3 in high dose, 1 reduced dose). The reduction in dose and addition of pneumocystis prophylaxis did seem to mitigate but not completely eradicate these events.
DISCUSSION
So what does this mean? This is the largest RCT in IgAN, with >500 participants with biopsy-proven disease and proteinuria (>1g/day). It demonstrated that oral methylprednisolone therapy for 6-9 months (full or reduced dose) significantly reduced the composite outcome of decline in kidney function, kidney failure or death due to kidney disease over a median follow-up of 4.2 years.
The strengths of this study were in its double blind randomization, stringent inclusion criteria and large sample size. However, of the 503 participants involved in the analysis only 431 individuals were identified as “completing the full treatment course” (20 did not receive the randomized therapy, 52 discontinued prematurely), and an additional 10 were lost to follow-up.
Were there other limitations or concerns? The most significant concern is related to the SAE associated with methylprednisolone therapy. Though the trial had been suspended for 14 months due to safety concerns, we only get 3 sentences in the main paper on adverse events. Severe infection requiring hospitalization, gastroesophageal bleeding requiring hospitalization, and death/permanent disability are devastating events, particularly in a young (median age 36), otherwise well cohort. Steroids have many other badness, and they reported no data on quality of life related to steroid therapy, including no information on weight gain, impaired glucose tolerance, appearance change, striae, blood pressure, insomnia, or mood disturbance: side-effects that are highly salient to our patients.
The changing sample size and protocols is worthy of some thought. The original sample size of 1500 was cut in half to 750 based on a change in endpoint (50% GFR loss to 40% GFR loss), as well as a revised estimate of event rate. Then the pause happened - for legitimate reasons given higher infection and death rates. The appropriate action was taken, with a lower steroid dose and the addition of antibiotic prophylaxis (with a slight change in GFR inclusion also: 30 instead of 20 as a lower limit). And the final sample size was also revised down to 500 - due to feasibility etc. But this does place an element of caution. The investigators took the best possible course of action, needless to say - but this is not a clean and blemishless sprint to the finish line for steroids.
In contrast to this stumbling path taken by steroids, the SGLT2i are hopefully cruising on smooth highways in IgAN. The DAPA-CKD RCT (Heerspink et al, NEJM 2020; NephJC summary by Priti Meena) compared the effect of dapagliflozin on a similar composite outcome of decline in eGFR of 50%, end stage kidney disease or death from kidney disease in individuals with CKD. The trial stopped early due to overwhelming efficacy. Of the >4000 participants enrolled, 270 had IgAN (254 biopsy proven). In this subgroup the results were equally clinically important. Dapagliflozin was seen to reduce proteinuria by 26%, resulted in a slower rate of eGFR decline (3.5 vs 4.7ml/min/1.73m2/year) and six (4%) on dapagliflozin vs 20 (15% on placebo) reached the composite endpoint over a median 2.1 years follow-up (Wheeler et al, Kidney Int 2021). Equally as impressively and as in the main trial, the safety profile was such that more participants discontinued the placebo than the study drug. So one could easily argue that unfortunately TESTING 2.0 is already out of date at time of publication, as flozins have now become part of baseline standard of care.
Another concern is the evidence that the benefits from methylprednisolone may diminish over time (as was the case for proteinuria) suggesting a delay but not avoidance of the primary outcome. A delay in need for dialysis or transplant is not something to frown upon; however this therapy comes at a cost. The delay in progression is encouraging as it suggests immunosuppression can confer a benefit, and we just need a more targeted, better tolerated therapy for this cohort. The lack of longer term benefit from steroid has been shown in other studies too, with the 10-year follow-up of the STOP-IgA cohort showing no difference in key clinical outcomes in individuals with IgAN randomized to immunosuppression versus supportive care arms (Rauen et al, Kidney Int 2020).
Other limitations of the study included that the majority of participants were from China, decreasing the trial's impact from a global perspective. IgA nephropathy is known to have a geographic variation. The underlying incidence, genetic factors, pathologic appearance, course - everything suggests that outcomes are worse in Asian, and particular Chinese, ethnicity. Indeed, the largest prior IgAN trial of steroids which was negative (STOP-IgA) was flat out negative - with a significant proteinuria reduction, but no GFR benefit. With 307 patients with similar eGFR (~ 60) and slightly lower proteinuria (~ 1.7g versus 2g in TESTING) one of the significant differences was that STOP-IGA was in a completely White population. TESTING only had 25 White patients. Other things - e.g. tonsillectomy - have had positive findings from Asia and are not practiced elsewhere.
CONCLUSION
This trial demonstrates a benefit of oral glucocorticoid therapy in patients with high risk IgAN within 5 years of administration. But… at a price. The morbidity and mortality related to this trial are a high cost in any population, but particularly a young one. Simply put, the burden of steroid therapy seems to outweigh any unsustained benefit.








Coming up next we have the TESTING RCT (again), with updated results on methylprednisolone treatment for IgA nephropathy.