#NephJC chat
Tuesday August 29th 9 pm Eastern
Wednesday August 30th 8 pm BST, 12 noon Pacific
JAMA. 2017 Aug 1;318(5):432-442. doi: 10.1001/jama.2017.9362.
Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial.
Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V; TESTING Study Group.
PMID: 28763548 Free Full Text Link courtesy JAMA
Background
IgA Nephropathy (IgAN) has varied clinical presentations from asymptomatic microscopic hematuria to rapidly progressive crescentic glomerulonephritis. Almost 30% of patients ultimately progress to ESKD within 20 years. Geographical variability in long-term outcome of IgAN is partly explained by lead-time bias and inclusion of milder cases in centers with good outcomes. Conservative treatment has proven to be very effective in IgAN, as shown in STOP-IgAN trial, where almost one third of patients who completed intensive optimization of conservative therapy over 6 months period, no longer qualified for randomization to the immunosuppressive therapy arm of the trial.
The pathogenesis of IgAN involves deposition of IgA1-containing immune complexes within the glomerular mesangium inciting an inflammatory response leading to renal damage. Hence, the use of corticosteroids as anti-inflammatory drugs makes sense. Previous studies by Pozzi and Manno et al have shown better renal survival with 6 month courses of corticosteroid in patients with IgAN. Both trials reported mild steroid side effects with no deaths.
Contrary to these studies, the more recent STOP-IgAN trial (see #NephJC coverage here) showed a reduction in proteinuria with steroids, but no improvement in renal survival. More importantly, the trial showed significantly increased risk of gastrointestinal and respiratory infections in the steroid arm of the trial.
In light of this conflicting evidence, the TESTING trial was designed to evaluate if corticosteroids, along with optimal supportive therapy, could safely prevent chronic kidney disease in patients with IgA nephropathy at risk of progression.
Methods
Setting: 262 patients from 2 countries (China and Australia). They initially planned to enroll 750 patients across 5 countries (China, Australia, India, Canada, and Malaysia), but this did not happen due to the premature termination of the study.
Design: Investigator-initiated, multi-center, double-blind, placebo-controlled, randomized clinical trial.
Inclusion criteria
Biopsy proven IgAN
eGFR between 20 and 120 mL/min/1.73 m2 (calculated using the CKD-EPI equation)
Urinary protein excretion greater than 1 g/d after 3+ months of maximum tolerated ACE/ARB
Exclusion criteria
Age less than 14 years
GFR less than 20 mL/min/1.73 m2
A strong indication (eg crescentic IgA) or contraindication (eg active infections) for corticosteroids
Systemic immunosuppressive therapy in the previous 1 year
Malignant /uncontrolled hypertension(>160mmHg systolic or 110mmHg diastolic)
Current unstable kidney function for other reasons, e.g. macrohaematuria induced acute kidney injury (past episodes were not a reason for exclusion)
Primary end-points
first occurrence of a 40% decrease in eGFR (initially was 50% but revised to 40% in Nov 2014 prior to knowledge of any study results), the development of ESKD or death due to kidney disease.
Secondary end-points
Secondary end points included :
The composite of ESKD, 40% decrease in eGFR, and all-cause death
The composite of ESKD, 50% decrease in eGFR, and all-cause death
Each of ESKD, death due to kidney disease, and all-cause death
Proteinuria reduction evaluated by time-averaged proteinuria and eGFR slope across all post-randomization study visits.
Intervention
There was a 3-month run in period where participants received maximally tolerated RAS blockade with optimal blood pressure control. Participants who continued to have urinary protein excretion greater than 1 g/d were randomized to
Placebo along with supportive care
versus
0.6 to 0.8 mg/kg/d of oral methylprednisolone (a maximal dose of 48 mg/d) for 2 months, then tapered by 8 mg/d each month, with a total treatment period of 6 to 8 months.
The trial was funded by the National Health and Medical Research Council of Australia, the Peking University Health Central Clinical Research Project, and the Canadian Institutes of Health Research. Study drug was provided by Pfizer Pharmaceuticals.
Results
262/523 potentially eligible patients underwent randomization (136 to methylprednisolone, 126 to placebo) between May 2012 and November 2015. The study was stopped early on advice from the Data Safety and Monitoring Committee (David Jayne, Mike Walsh, Tom Greene and Angela Wang) in November 2015 due to an excess of serious adverse events (SAE) in the steroid arm, mostly infectious in nature. 226 patients had completed the intervention by the time the study was terminated.
The two groups were pretty similar at randomization with predominantly Chinese population. Though the mean eGFR was 59.4 mL/min/1.73m2, majority (61.5%) had eGFR more than 50%. The average 24-hour urine protein excretion was about 2.5 grams/day.
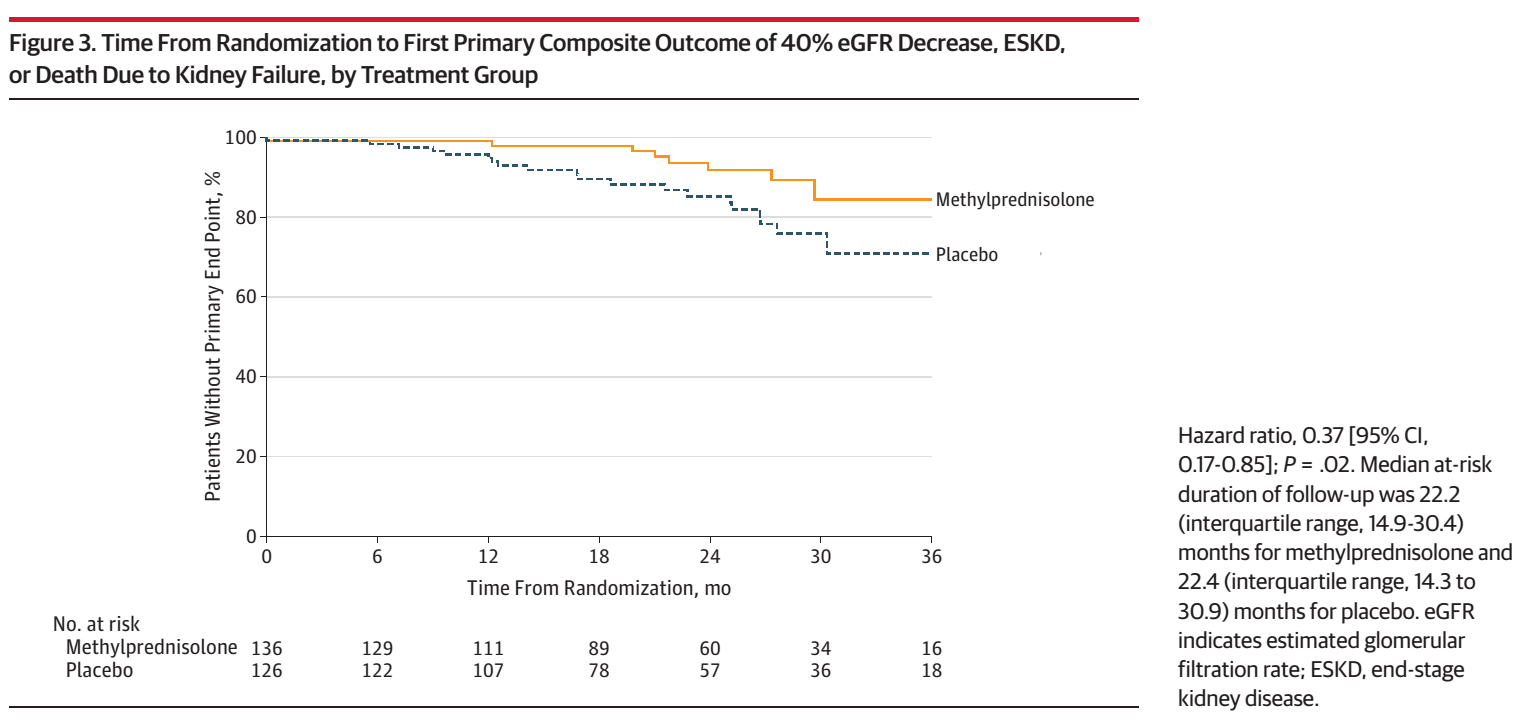
After a median follow-up of 2.1 years, the primary outcome occurred in 8 patients in the corticosteroid group vs 20 patients in the placebo group (5.9% Vs 15.9%, p = 0.02), which includes 4 (2.9%) vs 10 (7.9%) ESKD events (P= 0.10). The mean annual rate of eGFR decline was −1.79 mL/min/1.73 m2 in the methylprednisolone group, compared with −6.95 mL/min/1.73 m2 in the placebo group ( p = 0.03).
However, serious adverse events occurred in 20 patients (14.7%) in the corticosteroid group vs 4 patients (3.2%) in the placebo group (P= .001), largely due to an excess of serious infections (11 [8.1%] vs 0, P< .001), including 2 infection-related deaths.
Figure 3 from Lv et al, JAMA 2017
Conclusion
The authors concluded that steroid use in IgAN patients with proteinuria more than 1g/d was potentially beneficial for the kidney, but also predisposed to increased risk of predominantly serious adverse effects. However, definite conclusions regarding renal benefits could not be conclusively drawn due to premature termination of the trial.
Discussion
In this trial participants, steroid use reduced risk of kidney failure by almost 3 times while predisposing to 5 times increased risk of infectious serious side effects. However, premature termination of the trial, short follow-up period of 2.1 years, and questionable applicability of the results outside of Chinese ethnicity are major limitations.
The results of this trial contrast with the results of STOP-IgAN trial where steroid treatment did not reduce the risk of kidney failure. The authors cite following possible reason for this discrepancy:
higher risk participants in this trial as seen in the higher baseline proteinuria levels ( > 1 g/d Vs > 0.75 g/d in STOP-IgAN trial)
Perhaps individuals of Eastern Asian origin (eg, China) may have more rapid rates of eGFR decline and kidney failure
Higher rate of eGFR loss among patients in the supportive-therapy group of this study than that in STOP-IgAN (−6.95 vs −1.6 mL/min/1.73 m^2 per year)
Follow-up was longer in STOP-IgAN trial (median, 3.0 vs 1.5 years)
However, STOP-IgAN trial also reported higher number of infectious SAE in the immunosuppression arm.
So there is potential benefit - but definite harm, which is why the study was terminated early. This has led to the low-dose TESTING trial, which is now recruiting.
Also read the interesting JAMA editorial on this trial titled “TESTING for Benefit, Discovering Harm”