#NephJC Chat
Tuesday Feb 7 2022 9 pm EDT
Wednesday Feb 8 2022 9 pm IST
JAMA Jan 17;329(3):214-223. doi: 10.1001/jama.2022.23924.
Effect of Torsemide vs Furosemide After Discharge on All-Cause Mortality in Patients Hospitalized With Heart Failure: The TRANSFORM-HF Randomized Clinical Trial
Robert J Mentz, Kevin J Anstrom, Eric L Eisenstein, Shelly Sapp, Stephen J Greene, Shelby Morgan, Jeffrey M Testani, Amanda H Harrington, Vandana Sachdev, Fassil Ketema, Dong-Yun Kim, Patrice Desvigne-Nickens, Bertram Pitt, Eric J Velazquez; TRANSFORM-HF Investigators
PMID: 36648467
Introduction
Furosemide has been winning the hearts (and kidneys) of doctors everywhere since the ‘60s with its fast and effective natriuresis. Three decades later, torsemide, the new kid on the block, is claiming to be more potent and longer lasting. The generational battle continues. The irony is that we've come full circle - from the crude method of whole blood removal via leeches to the sophisticated technology of torsemide, only to find that the end goal remains the same: to relieve fluid overload. And yet, we're still left with the dilemma of choosing between furosemide and torsemide, like a never-ending loop.
But how do these drugs work, and what makes them different? Both furosemide and torsemide are loop diuretics, which block sodium chloride reabsorption in the thick ascending limb of the loop of Henle. This is achieved by inhibiting the Na-K-2Cl symporter in the luminal membrane. The differences reside in their pharmacodynamic properties, furosemide being known for its fast onset and short duration, while torsemide boasts of higher potency and longer effect.
Figure: Furosemide vs Torsemide (inspired by one of Joel’s tweets)
Furosemide is the most popular diuretic for patients with heart failure (HF). In a HF trial with more than 200,000 participants, furosemide was administered to 87%, while torsemide was administered to only 0.4% (Bikdeli B, et al, JACC 2013). However, torsemide might have some benefits over furosemide in HF, including a reduction in myocardial fibrosis, aldosterone production, and promotion of reverse ventricular remodeling (Peters AE, et al, Expert Rev CV Ther 2022). Torsemide may reduce aldosterone activity by blocking the aldosterone receptor and preventing the hormone's release. Additionally, torsemide's impact on the regulation of neurohormonal processes may contribute to the decrease in renin-angiotensin-aldosterone system (RAAS) activity (Buggey J. et al, Am Heart J 2015, Kasama S, et al, 2006). Conversely, both loop diuretics may have a detrimental impact on HF by upregulating the RAAS, which contributes to the progression of the disease and result in unfavorable outcomes for those taking higher doses of the diuretic.
So, we have physiological reasoning, but does this translate into hard endpoints? In two unblinded, comparative effectiveness trials from the early 2000s, Murray et al in the US, and Muller et al in Switzerland, compared torsemide and furosemide and showed no significant difference in mortality in patients with heart failure. Murray's study showed a reduction in HF and cardiovascular hospital readmissions, while Muller’s study showed improved quality of life for torsemide patients. The TORasemide In Congestive Heart Failure (TORIC) study, the largest previous trial, reported an improvement in NYHA functional class, a 59.7% relative risk reduction in cardiovascular mortality, and a 51.5% relative risk reduction in overall mortality in the torsemide group. However, the study was conducted primarily in rural Spain, and results may not be globally generalizable. The recent TORNADO study, with a small sample size and limited geographic scope, showed a statistically significant improvement in a composite outcome of NYHA class, a 6-minute walk test, and decrease in fluid retention.
There have been several meta-analyses conducted in recent years to collate data on the effectiveness of torsemide vs furosemide. These meta-analyses have varied in terms of methodology, sample size, and heterogeneity, including the number of studies involved (ranging from 2 to 19), and whether only randomized studies or a mix of randomized and observational studies were included. Despite these variations, some key findings have been consistently observed. Firstly, there is no significant difference in overall mortality between torsemide and furosemide treatment, although there is often high heterogeneity. Only one analysis, with a particularly talented and handsome cohort of authors, focused on cardiovascular mortality and found it significantly lower in torsemide-treated patients, based on data from just three studies (Abraham B, et al, Am J Cardiol 2020). Secondly, the majority of meta-analyses showed improved NYHA functional class in patients treated with torsemide, with low heterogeneity, except for an early analysis from 2013 (Bickdeli B, et al, JACC 2013). There were inconsistent conclusions regarding hospitalization rates, with three meta-analyses showing improvement in HF re-hospitalization rates and two showing improvement in HF or cardiovascular re-hospitalization, and Abraham et al found only a non-significant but lower risk of HF hospitalization with torsemide treatment.
Adapted from Peters AE et al, 2022
Now that we've gone the extra mile, diving into a meta-analysis of meta-analyses, it's time to tackle the age-old question in heart failure treatment: is it torsemide or furosemide that reigns supreme?
The Study
Methods
TRANSFORM-HF was a pragmatic, open-label, randomized trial that aims to compare the efficacy of furosemide and torsemide in treating HF. The pre-specified hypothesis was that torsemide would reduce all-cause mortality by 20% compared to furosemide.
Population
This trial included the whole spectrum of HF. To be eligible for the trial, patients had to have have been hospitalized for at least 24 hours due to worsening of chronic heart failure or a new diagnosis of heart failure and meet one of two criteria - a LVEF of ≤40% within 24 months prior to the hospitalization or elevated natriuretic peptide levels during the hospitalization.
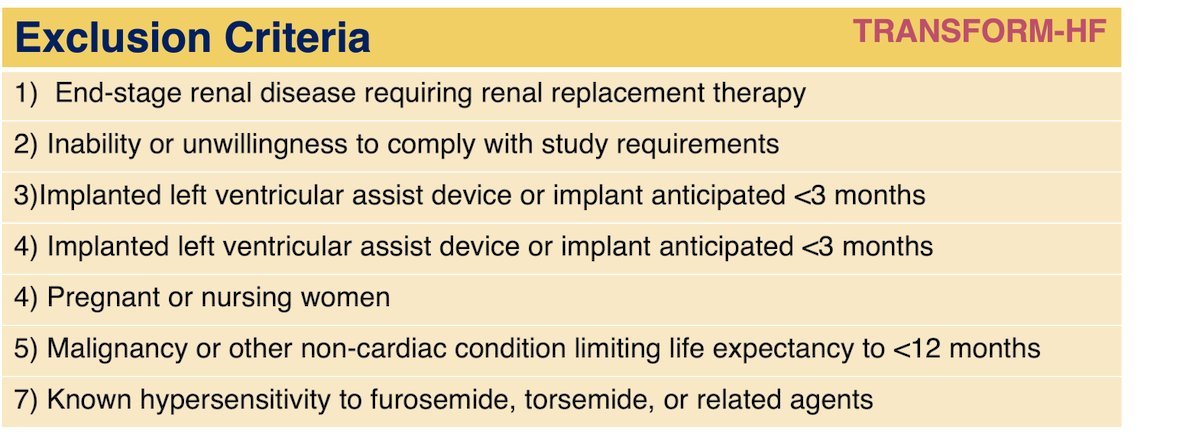
Here are the inclusion and exclusion criteria:
Recruitment occurred from June 2018 through March 2022, with follow-up through 30 months for death and 12 months for hospitalizations. The final date for follow-up data collection was July 2022.
Interventions
During hospitalization, after providing informed consent, participants were randomized 1:1 (according to CONSORT diagram) to receive torsemide or furosemide as their diuretic treatment strategy prior to hospital discharge. The study used a dose conversion of 1 mg of torsemide for every 2/4 mg of oral furosemide. Changes in dose and frequency of the randomized therapy after discharge were at the discretion of the patient’s outpatient clinicians. The follow-up for the study was conducted through a centralized system managed by the Duke Clinical Research Institute. Participants were contacted by phone at various intervals (30 days, 6 months, and 12 months after discharge).
Outcomes
Primary outcome was all-cause mortality in a time-to-event analysis. Secondary outcomes were all-cause mortality or all-cause hospitalizations (over 12 months), total hospitalizations (over 12 months), and all-cause mortality or all-cause hospitalizations (over 30 days).
Statistics and Analysis
The sample size for the trial was calculated based on previous observational data and a meta-analysis of mortality rates with torsemide vs furosemide, taking into account the goal of detecting a 20% reduction in death events with torsemide (Bikdeli B, et al, 2013). At least 721 primary endpoint events (all-cause mortality) were needed for the trial to have 85% power to detect a hazard ratio of 0.80, assuming 1:1 randomization, and it was estimated that up to 6000 participants would be required to achieve this goal. The sample calculation did not include the potential impact of additional variables on the primary outcome.
Funding/ sponsor
TRANSFORM-HF was supported through cooperative agreements from National Heart, Lung, and Blood Institute. NHLBI had no role in the design of the study, but NHLBI staff did participate in the study conduct, collection, management, analysis, and interpretation of the data.
Results
TRANSFORM-HF included 2859 patients who were hospitalized for heart failure and had a median follow-up period of 17.4 months.
Figure 1 from Mentz et al, JAMA 2023
The trial had a diverse participant population, with 36.9% of participants being women and 33.9% being Black individuals. Approximately 70% of participants had a left ventricular ejection fraction of less than 40%. Either N-Tpro-BNP or BNP levels could be used for inclusion, with median levels of 3900 pg/ml and 950 pg/dL respectively. Approximately one-third of participants had chronic kidney disease, with mean estimated glomerular filtration rate of 59 mL/min. In terms of medication, only 40% of patients were taking angiotensin-converting enzyme inhibitors, 35% were on mineralocorticoid receptor antagonists, 19% were on sacubitril-valsartan, and 6% were on sodium-glucose cotransporter 2 inhibitors.
Table 1 from Mentz et al, JAMA 2023
Adherence to Trial Meds
Nearly 90% of patients (2491 out of 2755) with known prescription status at hospital discharge were taking their assigned loop diuretic. At discharge, 7% switched from torsemide to furosemide, and 3.8% switched from furosemide to torsemide (5.4% total). By 30 days and 6 months, 7% and 9.5% were no longer taking any loop diuretic, respectively.
Loop Diuretic Dosage A Match Made in... Hospital?
At discharge, the mean loop diuretic dose was pretty much the same in both groups (79.3 [63.3] mg in furosemide equivalents). But fast forward a month and things got a little peculiar- the torsemide group was taking a higher dose (77.8 [74.5] mg) than the furosemide group (68.4 [50.2] mg).
Outcomes
In the study, 373 of 1431 patients in the torsemide group and 374 of 1428 patients in the furosemide group died, resulting in a death rate of 26.1% and 26.2%, respectively. This difference was not statistically significant (P = 0.76). The effect of torsemide on the primary outcome was the same across different subgroups of patients and the results remained consistent in sensitivity and post hoc analyses, including adjustments for COVID-19.
Figure 2 from Mentz et al, JAMA 2023
At 12 months, the composite of all-cause mortality or all-cause hospitalizations occurred in 47.3% of patients in the torsemide group and 49.3% of patients in the furosemide group, with a hazard ratio of 0.92 (95% CI, 0.83-1.02). Additionally, 37.5% of participants in the torsemide group and 40.4% of participants in the furosemide group experienced any hospitalization, with a rate ratio of 0.94 (95% CI, 0.84-1.07).
Table 2 from Mentz RJ, et al, JAMA, 2023
More Analyses
Despite these similarities, a post hoc analysis showed that the risk of all-cause hospitalization over 12 months was slightly lower for the torsemide group, with a hazard ratio of 0.88 compared to the furosemide group. These findings indicate that, while both medications may have similar effects on overall health outcomes, there may be a slightly lower risk of hospitalization for patients taking torsemide. This is an intriguing finding, as hospitalization is often a pressing concern for patients and healthcare providers. How can we explain the wizardry behind studies’ results and post hoc analysis when coming up to total hospitalizations? The original analysis showed negative total hospitalizations, but a post-hoc analysis with a different method, the Fine and Gray competing risk model, showed different results. This model took into account factors such as age, sex, baseline ejection fraction, and prior diuretic treatment, as well as considering death as a competing event.
Table 2 from Mentz RJ, et al, JAMA, 2023, Supplement 3
Discussion
The big question in Nephrology (and Cardiology) circles: which loop diuretic will we choose? The TRANSFORM-HF trial aimed to shed light on the matter, but unfortunately, we're still in the dark. With a smaller sample size than expected and a higher rate of patient withdrawals, the results just aren't robust enough.
The study's design and methodology presented limitations, such as using all-cause outcomes that may not have accurately captured heart failure-specific outcomes and a deviation from conventional clinical adjudication methods in classifying endpoints. All-cause endpoints may have been insensitive to detecting a potential treatment effect, and cardiovascular-specific outcomes are not reported.
The number of participants in the study was less than expected, compromising the strength of the data from the sub-group analysis. Additionally, the rate at which patients withdrew was greater than expected, potentially due to limited contact with the research site.
The treatment effect was based on pre-existing meta-analyses, which may have overlooked the advantages of therapies recommended by guidelines, particularly for patients with reduced ejection fraction heart failure. In addition, a significant proportion of participants had newly diagnosed heart failure, so changes in guideline-directed medical therapy post-baseline (such as the addition of ARNI, β-blocker, SGLT2 inhibitor, and mineralocorticoid receptor antagonist) could have impacted the clinical outcomes. Unfortunately, the pragmatic design of the study did not allow for the evaluation of the relative benefits and drawbacks of the treatments, such as changes in kidney function, electrolyte imbalances, or non-hospitalization events.
Consistent with the pragmatic design, the trial facilitated rapid recruitment of a large, diverse population (n = 2,859, 37% women, 34% Black race, 46% HFmrEF/HFpEF). The pragmatic nature of the trial is reflected in its approach to centralized follow-up through telephone interviews rather than in-person visits. The focus on practical considerations, such as assessing treatment adherence, vital status, and hospitalization events, as well as gathering data through readily available sources like hospital records and the National Death Index, highlights the trial's commitment to real-world applicability. The event-driven design and target enrollment of 6000 patients further support the pragmatic focus of the trial.
Loop diuretic discontinuation (7.5% in torsemide arm and 6.5% in furosemide arm at 30-days) and early crossover (8% in torsemide arm and 5.5% in furosemide arm at 30-days) during follow-up may have influenced the results towards neutrality, possibly due to bias from patients or clinicians regarding the differential benefits of loop diuretics. The study's results were also potentially impacted by the discretion given to clinicians in loop diuretic dosing. Future research should examine the impact of non-adherence and dose titration on the findings, evaluate alternative definitions of "as-treated", and incorporate time-varying factors. The low recruitment of patients with heart failure with preserved ejection fraction and those of Hispanic ethnicity were also limitations in this study.
The TRANSFORM-HF trial made us realize that we are stuck in a loop with loop diuretics, doggedly expecting different results despite the same outcome. It's a never-ending cycle that we need to break out of and look for drugs that provide real CV benefits and truly transform the lives of our patients.
Conclusion
The results of the comparison between torsemide and furosemide in post-hospitalization heart failure patients were underwhelming, with no significant difference in all-cause mortality observed over the course of 12 months. However, the validity of these conclusions is hampered by the issues of participant dropout, crossover, and medication non-adherence. Thus, a more definitive answer to the question of the efficacy of these drugs remains elusive.
Summary prepared by
Cristina Popa, MD,
Department of Internal Medicine-Nephrology,
University of Medicine and Pharmacy
"Grigore T Popa", Iași, Romania
Reviewed by Jade Teakell and Jamie Willows
Featured image credit: Artificial Intelligence directed by Evan Zeitler