JAMA. 2025 Oct 29:e2520231. doi: 10.1001/jama.2025.20231. Online ahead of print.
Sodium Bicarbonate for Severe Metabolic Acidemia and Acute Kidney Injury: The BICARICU-2 Randomized Clinical Trial
Boris Jung, Mathieu Jabaudon, Audrey De Jong, Laurent Bitker, Jules Audard, Kada Klouche, Benjamine Sarton, Christophe Guitton, Sigismond Lasocki, Benjamin Rieu, Emmanuel Canet, Caroline Jeantrelle, Antoine Roquilly, Julien Mayaux, Franck Verdonk, Julien Pottecher, Martine Ferrandiere, Beatrice Riu, Pierre Garcon, Mona Assefi, Philippe Detouche, Jean Marie Forel, Claire Roger, Jeremy Bourenne, Sophie Jacquier, David Bougon, Amelie Rolle, Philippe Corne, Nacim Benchabane, Jean Christophe Richard, Karim Asehnoune, Gerald Chanques, Jean Reignier, Foud Belafia, Maxime Fosset, Helena Huguet, Emmanuel Futier, Nicolas Molinari, Samir Jaber; BICARICU-2 Study Group
PMID: 41159812
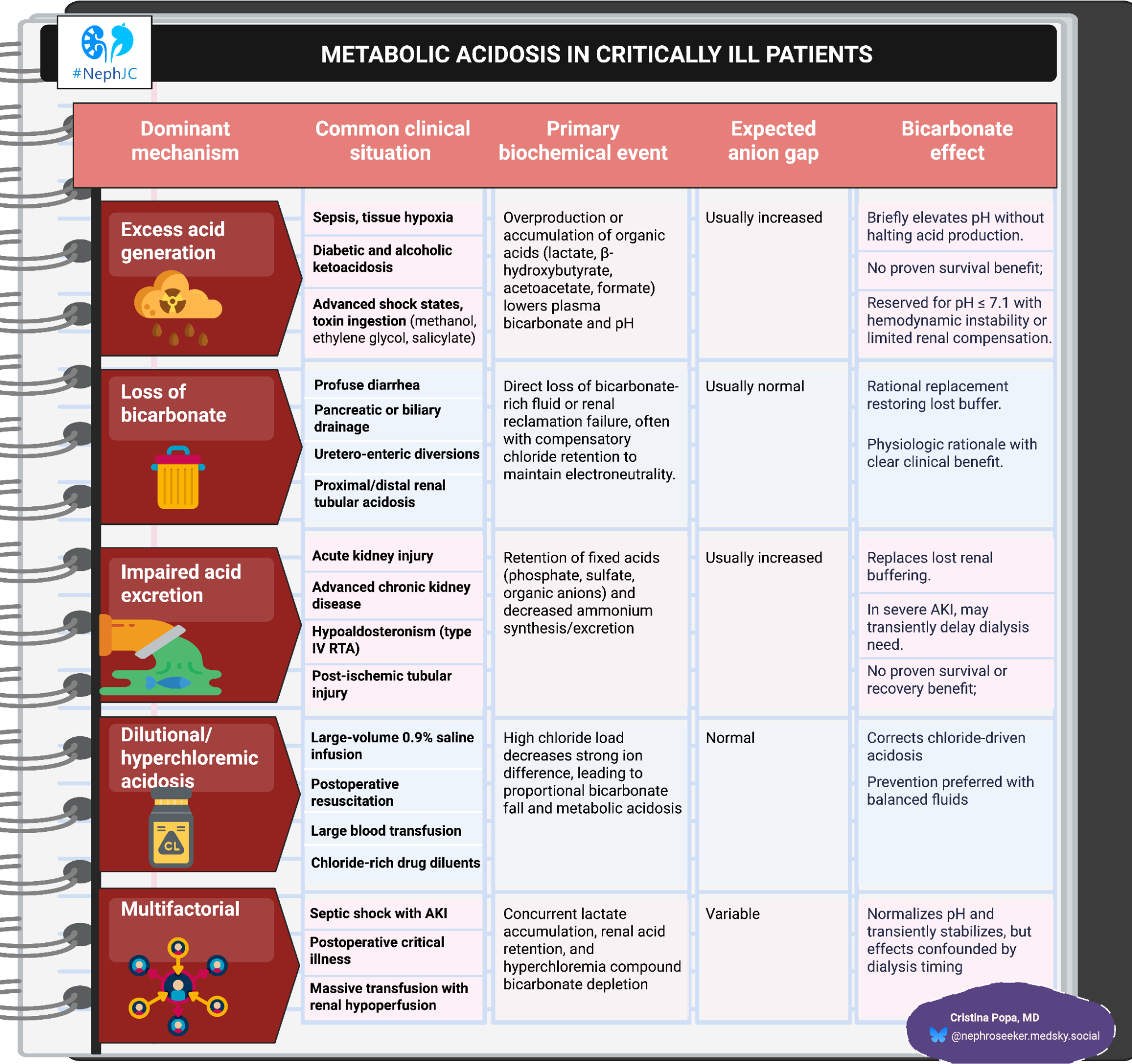
Metabolic acidosis in the ICU: mechanism
Metabolic acidosis in the ICU is rarely a single-etiology event; it stands at the crossroad of impaired buffering, acid overproduction, and renal dysfunction under extreme physiologic stress. The typical academic ICU patient manifests as a combination of high-anion-gap acidosis (HAGMA) from lactate or unmeasured anions, and non-anion-gap acidosis (NAGMA) driven by chloride accumulation or bicarbonate loss. (Kraut JA, Nat Rev Nephrol. 2012)
The physiological consequences are profound once the arterial pH falls below 7.20. Acidemia impairs cardiac contractility by reducing myofilament calcium sensitivity and blunts vascular responsiveness to catecholamines, promoting hypotension and arrhythmias. It can also suppress immune function while paradoxically stimulating a pro-inflammatory state.
In acute kidney injury (AKI), the kidney’s role in acid-base homeostasis collapses: retention of phosphate, sulfate, and organic acids and reduced ammoniagenesis compound the acid load. (Wardi G, et al. J Emerg Med. 2023)
The mechanistic logic for bicarbonate use is straightforward: replace the missing buffer, correct extracellular pH, and restore cardiovascular reactivity. But sodium bicarbonate also generates CO2, decreases ionized calcium, and expands extracellular volume, which may offset hemodynamic benefit. Historical overuse, particularly during cardiac arrest and isolated lactic acidosis, failed to improve outcomes, leading to persistent skepticism. (Cooper DJ, Ann Intern Med, 1990| Kim HJ, PLoS One, 2013| El-Solh AA, Intern Emerg Med. 2010) A recent state-of-the-art review catalogues potential harms: paradoxical cerebrospinal fluid acidosis via CO2 flux, intracellular acidosis, left/right shifts of oxyhemoglobin curve, electrolyte shifts, and osmotic effects, while simultaneously acknowledging the heterogeneity of data linking these mechanisms to hard outcomes. (Eraky AM, J Clin Med. 2024)
From BICAR-ICU1 to BICAR-ICU2: refining the question
The first BICAR-ICU trial (Jaber et al, Lancet, 2018| NephJC summary), randomized 389 ICU patients with severe metabolic acidemia to open-label bicarbonate therapy (4.2% solution, target pH ≥7.30) or standard of care. The primary endpoint (28-day mortality or organ failure at day 7) was neutral, but subgroups with AKI stage 2-3 showed reduced mortality and less need for renal replacement therapy (RRT). This unexpected renal signal, coupled with the physiologic plausibility of buffer replacement in “uremic acidosis”, became the rationale for BICAR-ICU 2.
Figure 2A Time to death in the overall population, and 2B patients with prespecified acute kidney injury, and 2C the 28-day mortality risk difference in the overall population and in the three prespecified strata, from Jaber et al, Lancet, 2018
Several recent datasets reinforce this rationale. The international SODA-BIC study (Fujii T, Crit Care, 2021) documented wide heterogeneity in bicarbonate use, ranging from 5 to 78% of acidemic patients, despite similar severity and acid-base parameters. Practice was empiric, dosing unstandardized, and outcomes inconsistent. In a large retrospective cohort (Zhang Z, Intensive Care Med, 2018), no mortality benefit was observed overall, but improved survival in patients with AKI stage 2-3, and pH< 7.2 (HR 0.74, 95% CI 0.51-0.86). More recently, in MIMIC-III/IV cohorts, bicarb consistently showed no survival benefit in septic acidosis, but a favorable association when severe acidemia coexists with AKI stage 2-3. In AKI with high anion-gap acidosis, hospital mortality improved with a mid-range exposure (800-1600mL infused), an exploratory dose window that aligns with physiologic “replacement” rather than aggressive alkalinization. (Wang Y, Front Med (Lausanne). 2023) These data justified a confirmatory trial, not to cure AKI, but in nephrology terms, to test whether a “bag of base” can momentarily stand in for dialysis when acidosis is the only impetus for initiation.
BICAR-ICU2: design, limitations, and AKI
BICAR-ICU2 (Jung B, JAMA, 2025) enrolled 628 patients across 43 French ICUs, all with severe metabolic acidosis (pH≤ 7.20, bicarb≤ 20mmol/L) and KDIGO stage 2-3 AKI. Patients received open-label 4.2% sodium bicarbonate titrated to pH ≥ or standard of care. 90-day mortality was neutral (62.1% vs 61.7%).
Figure 2. A. Primary outcome: Cumulative incidence of death by day 90, and 2B. Secondary outcome: cumulative use of renal replacement therapy by day 28, from Jung B, et al, JAMA, 2025
Renal benefit rests almost entirely on a delay and reduction in RRT. Median time to RRT initiation was doubled with bicarbonate (31h vs 16h), producing a lower cumulative incidence of dialysis over 28 days (HR 0.59; 95% CI 0.46-.0.75). Yet, this difference reflects how clinicians responded to corrected acidemia more than how the kidneys recovered.
The main triggers for RRT- persistent oliguria (approx. 85% cases), refractory acidosis, and rising urea or creatinine - were similar between groups, but the frequency of acidosis driven dialysis fell sharply (41% vs 64%). In contrast, dialysis for oliguria or biochemical uremia was unchanged. The pattern shows that bicarb primarily neutralized one of the diagnostic entry points into RRT: low-PH altering renal physiology.
eTable 5. Frequency and reasons for RRT, from Jung B, et al, JAMA, 2025
Limitations likely to bias the renal conclusion:
Open-label with pH-based dialysis triggers. Low pH (≤ 7.15) was an indication for RRT. Bicarbonate directly alters the trigger, creating endpoint circularity.
Decision endpoint, not biologic endpoint. “Need for RRT” mirrors clinician behaviour, not nephron recovery.
Etiologic heterogeneity within AKI: septic, ischemic, and nephrotoxic injuries were pooled; responsiveness to buffering differs by mechanism.
Nonstandardized dose/ ventilation: titration pH, not base deficit; CO2 clearance not protocolized, allowing variable intracellular effects and ventilatory load. Contemporary reviews emphasize that clinical bicarbonate dosing is commonly not standardized, hampering causal inference (exactly what BICAR-ICU2 inherited from practice).
High mortality background (62%): any modest survival effect would be undetectable.
Interpretation and mechanistic context
Beyond neutral primary outcome and valid methodological critiques, the investigators emphasize less RRT with bicarbonate. That finding most likely reflects altered indications and timing for dialysis because acidosis is a principal reason; pharmacologic pH correction reduces crossing that threshold.
Mechanistically, bicarbonate is plausible when the problem is buffer deficit (uremic or hyperchloremic states), where replacement restores the extracellular buffer normally provided by functioning kidneys. Where acid overproduction dominates (i.e. isolated lactic acidosis), buffering does not address the cause.
Reassessing the magnitude of risk
Neuro-respiratory risks attributed to bicarbonate (paradoxical CSF acidosis through rapid CO2 diffusion across the blood-brain barrier, transient intracellular acidosis, and possible blood-flow effects) remain plausible but poorly sustained. (Eraky AM, J Clin Med. 2024) Evidence in adult critical care is inconsistent, largely extrapolated from small physiologic studies rather than outcome trials.
Recent comprehensive reviews converge on a pragmatic view: bicarbonate predictably alters blood chemistry (raising pH, PaCO2, high sodium, lowering potassium, and anion gap), but rarely translates these shifts into measurable hemodynamic or neurologic harm under controlled ventilation. (Yagi K, Crit Care, 2021) Beyond BICAR-ICU, randomized data remain sparse, leaving the debate suspended between physiology and empiricism.
The mirage of renal rescue
The BICAR-ICU2 story closes where the broader dialysis literature has already been circling: the tension between correcting numbers and changing outcomes. The lower rate of RRT in the bicarbonate arm mirrors AKIKI (Gaudry S, N Engl J Med, 2016), STARRT-AKI (STARRT-AKI Investigators, N Engl J Med, 2020| NephJC summary), and AKIKI-2 (Gaudry S, Lancet, 2021| NephJC), which taught that the timing of dialysis can be delayed safely, but not because physiology improves. Primarily, it is because of arbitrary, and often provider-specific, thresholds.
ELAINE stands as the outlier: a single-center pilot where early RRT seemed life-saving. (Zarbock A, JAMA, 2016| NephJC) Yet it's tightly controlled, homogenous cohort and universal dialysis exposure, making it more a proof of logistics. By contrast, AKIKI and AKIKI-2- large, pragmatic, French multicenter studies demonstrated that earlier initiation of RRT changes nothing about survival, and further postponement of dialysis remains largely safe. The endpoint shifts, not patients’ outcomes.
Reviewed by Brian Rifkin and Swapnil Hiremath