Lancet . 2025 Dec 13;406(10521):2782-2791.
doi: 10.1016/S0140-6736(25)01717-9. Epub 2025 Nov 13.
A preventive care strategy to reduce moderate or severe acute kidney injury after major surgery (BigpAK-2); a multinational, randomised clinical trial
Alexander Zarbock, Marlies Ostermann, Lui Forni, Christian Bode, Lennart Wild, Christian Putensen, Diego Parise Roux, Elena Elías Martín, Christian Arndt, Tim Rahmel, Silvia de Rosa, Céline Monard, Antoine G Schneider, Adam Glass, Mona Jung-König, Stefano Romagnoli, James Gossage, Nuttha Lumlertgul, Jan Gerrit Haaker, Javier Ripollés-Melchor, Savino Spadaro, Antonio Siniscalchi, Emmanuel Futier, Lucie Aupetitgendre, Irene Romero Bhathal, Raquel García Álvarez, Alice Bernard, Peter Rosenberger, Carola Wempe, Mahan Sadjadi, Melanie Meersch, Karen Fischhuber, Rinaldo Bellomo, John A Kellum, Thilo von Groote; BigpAK-2 study group
PMID: 41242333
Why was this trial done?
Acute kidney injury (AKI) after major surgery is common (up to 30–40%) and strongly associated with higher mortality, CKD progression, longer ICU and hospital length of stay, and higher costs (Boyer et al CJASN 2022). Over the last 15–20 years, multiple perioperative “nephroprotective” strategies (tight glucose control, remote ischemic preconditioning, renal vasodilators, CO-guided therapy) have mostly failed to show meaningful reductions in AKI. KDIGO 2012 gave us a supportive care bundle, but observational data show implementation is poor.
BigpAK-2 asks: if we identify high-risk patients using an early kidney stress biomarker (TIMP-2 x IGFBP7) and then apply a structured KDIGO prevention bundle, can we finally reduce clinically important (KDIGO 2–3) postoperative AKI?
What is this biomarker TIMP-2 × IGFBP7?
TIMP-2 and IGFBP7 are urinary markers of tubular cell-cycle arrest and early stress, rising hours before creatinine or urine output change (Kashani et al., J Trauma 2016, Sapphire Study; Bihorac et al., Am J resp Crit Care Med 2014 Topaz Study | NephJC summary). The product TIMP-2 x IGFBP7, reported as (ng/mL)²/1000, has been validated to predict moderate–severe AKI within the next 12–24 hours, and are marketed as ‘NephroCheck’. BigpAK-2 used a cutoff ≥0.3 to define high-risk patients eligible for randomisation. This is a well-described ‘enrichment’ strategy to include patients at high risk and reduce noise (Parikh et al, Kidney Int 2016).
KDIGO Prevention Bundle
The KDIGO bundle is essentially the 2012 KDIGO AKI prevention recommendations (grade 2C) packaged into a protocol:
Maintain MAP ≥ 65 mmHg
Assess fluid responsiveness using a passive leg raise (PLR) test
Give crystalloid boluses (500–1000 mL) only if PLR shows >10% increase in cardiac output
Use norepinephrine to treat hypotension when fluid unresponsive
Add dobutamine or epinephrine if cardiac index remains <2.5 L/min/m²
Avoid nephrotoxins (NSAIDs, aminoglycosides, amphotericin B, HES/gelatin, unnecessary contrast)
Hold ACE inhibitors and ARBs in the perioperative period
Target moderate glycemic control (≈100–150 mg/dL)
Avoid chloride-rich fluids
Reassess hemodynamics every 3 hours for 12 hours after randomization
eFigure 1: intervention arm protocol. Algorithm used in BigpAK-2 to deliver the KDIGO bundle: PLR-guided fluid therapy, cardiac index target ≥2.5 L/min/m², and MAP ≥65 mmHg supported with norepinephrine and inotropes, reassessed every 3 hours up to 12 hours.
How was this Study done and What did it show?
Methods
BigpAK-2 was a large, multinational, adaptive randomized controlled trial conducted across 34 centres in eight European countries. Adults (≥18 years) undergoing major surgery >2 hours and admitted postoperatively to ICU or high-dependency units were screened. Four hours after ICU/HDU admission, urine TIMP-2 x IGFBP7 was measured. Only patients with TIMP-2 x IGFBP7 ≥0.3 and at least one additional clinical AKI risk factor (age ≥75 years, vasopressor requirement, mechanical ventilation, CKD stage 3, or intraoperative contrast exposure) were randomised 1:1 to:
Intervention: KDIGO prevention bundle for 12 hours using the algorithm above
Control: usual postoperative care without structured bundle or mandated monitoring
Key exclusions included CKD stage 4–5 (eGFR <30), pre-existing AKI, anuria, recent RRT, pregnancy, and recent transplant. The primary endpoint was KDIGO stage 2–3 AKI within 72 hours, adjudicated by blinded outcome assessors. Secondary outcomes included any stage AKI, RRT, mortality, and MAKE90 (major adverse kidney events that occur within 90 days after AKI), as well as several safety outcomes.
Results
Of the ~ 8000 patients screened, ~ 1200 (just under 600 in each arm) were enrolled and randomised (see figure 1).
Figure 1 from Zarbock et al, Lancet 2025
Table 1 and 2 (below) show the kind of patients who were enrolled. The mean age was ~ 70 years with 2/3rd being men. The pre-op serum creatinine was ~ 0.90 mg/dL (~ 80 μmol/L). About 20% did have stage 3 CKD, and a quarter had DM. Almost 40% had a history of cancer. Notably, almost half the patients were on a RASi (ACEi or ARB) prior to enrollment. Almost all the surgeries were elective, and ~ one-third were “general or abdominal”, which is a higher percentage than historically expected to require admission to the ICU postoperatively.
Table 1 and 2 from Zarbock et al, 2025 Lancet
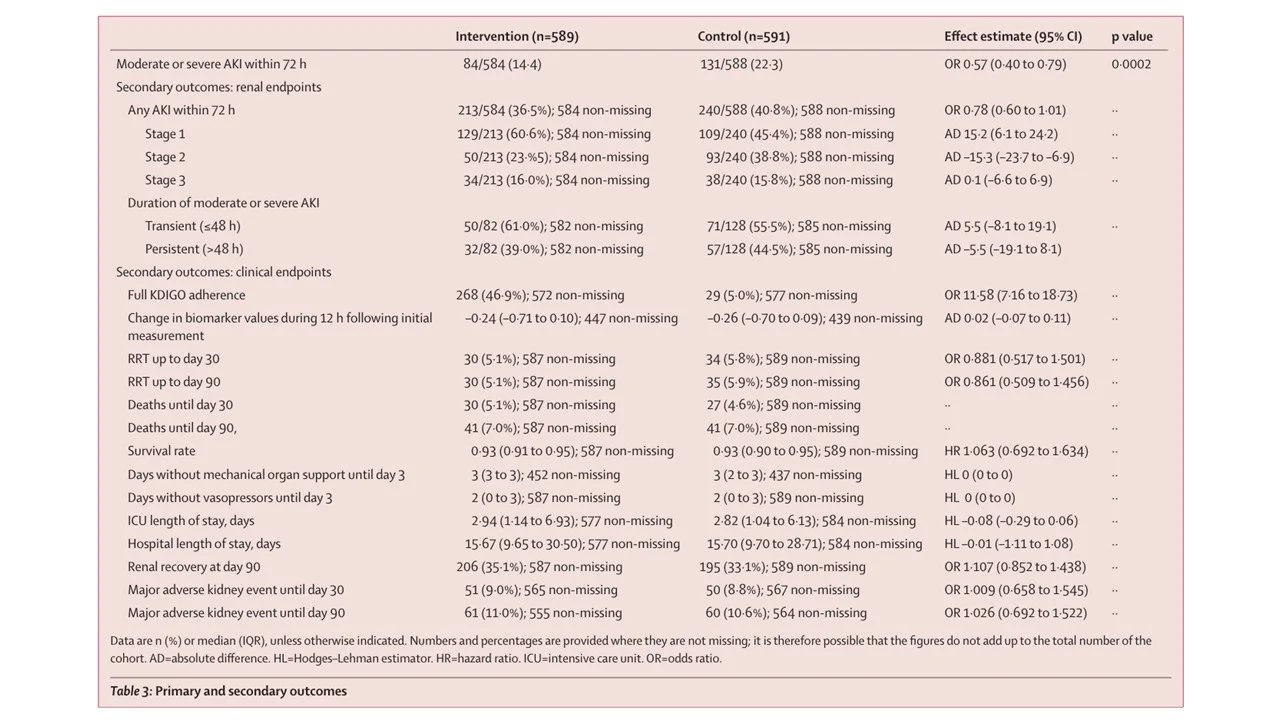
There was huge effect on the primary outcome, the occurrence of moderate or severe AKI within 72 h after surgery, assessed in the intention-to-treat population (HR 0.57, 95% CI 0.40 to 0.79) (see table 3 below). However, there was no significant difference between groups in any-stage of AKI (KDIGO 1–3), RRT at 90 days, mortality, or MAKE90. Interestingly, the change in the biomarker (Nephrocheck), which is a supposed to be a sensitive signal of kidney injury, was also not significant (-0.24 and -0.26).
Table 3 from Zarbock et al, 2025 Lancet
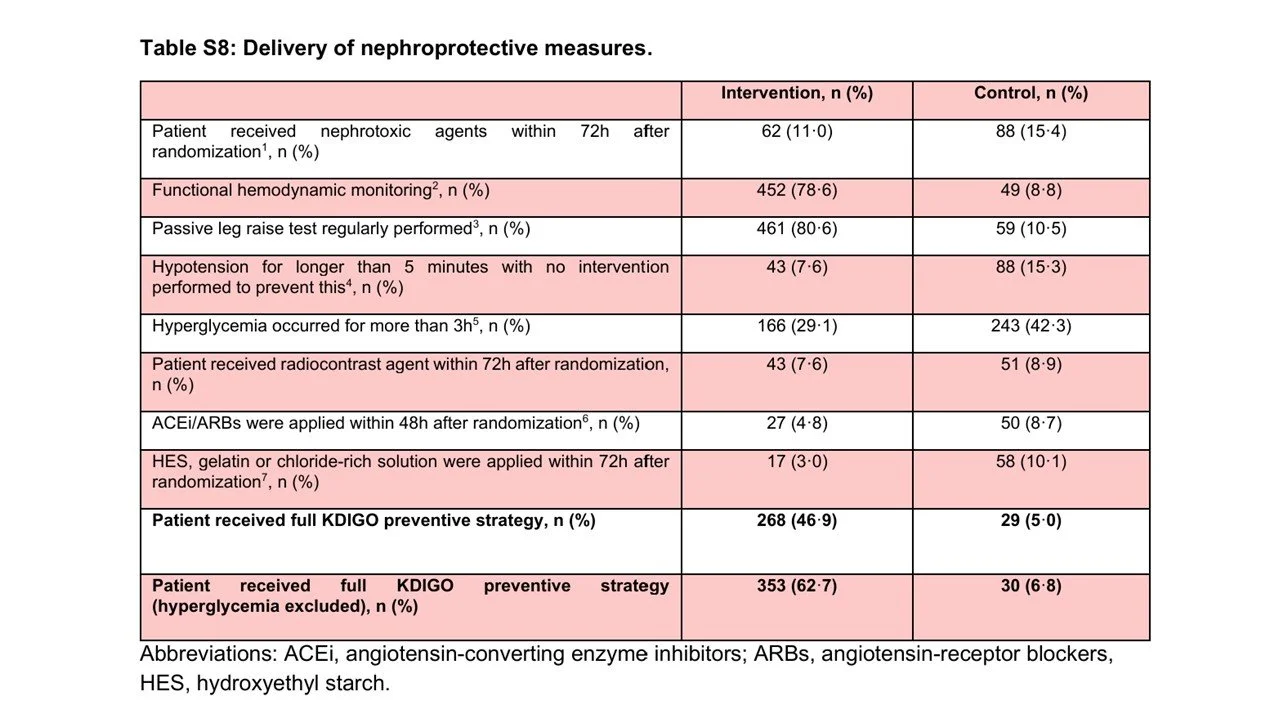
Delivery of Nephroprotective Measures
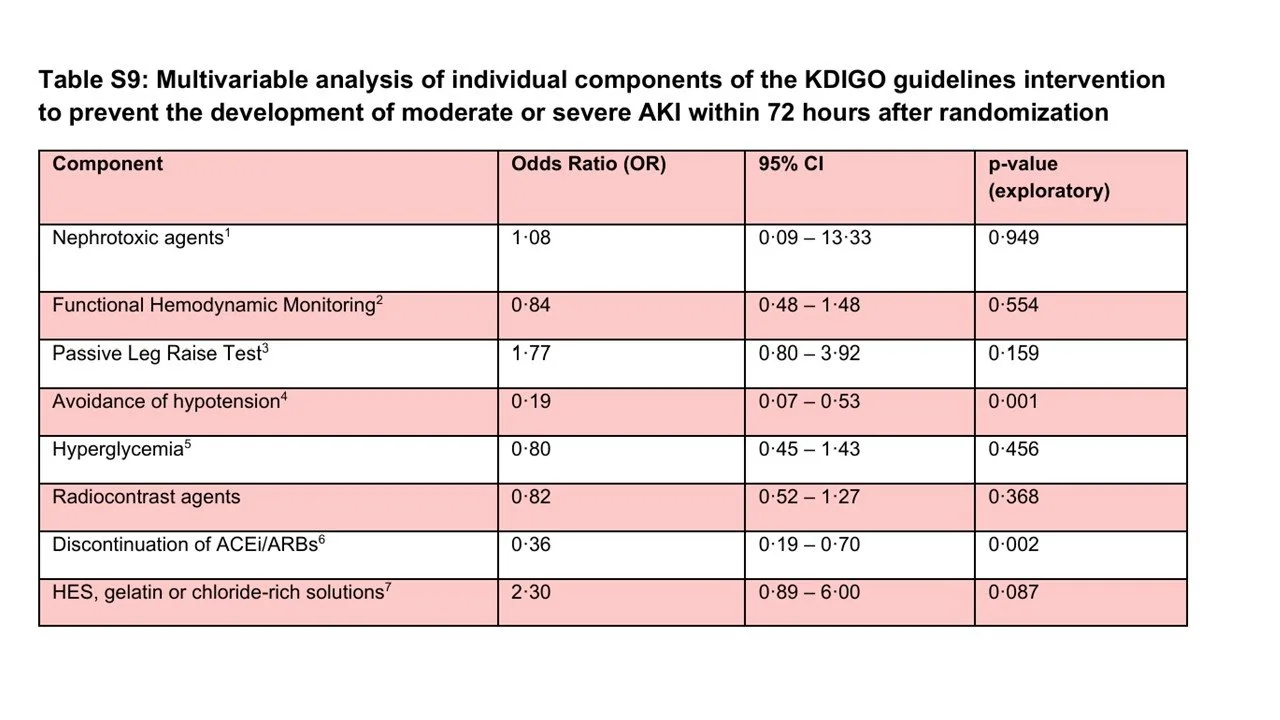
You have to dive deep into the supplement to see which components of the KDIGO prevention bundle were actually followed. Passive leg raising and functioning hemodynamic monitoring were often done – but the other components of the bundle were uncommonly implemented (see below). An exploratory analysis (table S9) suggests that the prevention of hypotension and discontinuation of ACE inhibitors and ARBs had the strongest association with the primary outcome.
Table S8 from Zarbock et al, 2025 Lancet
Table S9 from Zarbock et al, 2025 Lancet
What are the implications?
The authors would say that using biomarkers and a bundled approach is what made all the difference in the primary outcome – but is that really true? What we see here is that an intensive AKI preventative bundle, applied to sick patients at high risk of AKI, reduced their serum creatinine without changing need for RRT, ICU or hospital length of stay, or mortality. More importantly – the change in creatinine was not accompanied by a change in kidney injury biomarkers. So, what might be happening here? The fact that stopping RASi and avoiding hypotension were the two most significant interventions should give us pause that maybe these interventions are just changing serum creatinine via hemodynamic effects, without really altering the presence of true kidney injury. One could even question the preventive strategies employed. There exist at least some data (Brar et al JAMA IM 2018 | NephJC summary) suggests that continuing RASi through AKI is actually associated with better outcomes and AKI protection. Unfortunately, the positive leaning editorial in Lancet doesn’t discuss any of these confounding factors.
Should this bundle get elevated in status in the next version of KDIGO? It is certainly possible, since this RCT showed benefit in serum creatinine in this ICU population – but one can decide if any of this is clinically worthwhile. Stopping RASi and giving more IVF in the setting of a RCT, while lowering creatinine without altering dialysis or mortality, may leave pragmatists with reluctance and skepticism to adopt such a strategy. After all, we have been led astray by such trials in the past.
NephJC Shorts by Nawaf Alyahya, Nephrology Fellow, University of Ottawa, and Swapnil Hiremath
Reviewed by Brian Rifkin