First principles
Tolerance is not “less rejection”, it’s finding an immunological equilibrium. Two levers set it:
Central deletion: donor-reactive T-cells are removed during thymic education when donor antigen is continuously presented by donor-derived antigen-presenting cells (APCs).
Peripheral regulation: effector T helper cells (Th1, Th17, follicular Th) are restricted early, giving central deletion the time and space to work. When Tfh activity is suppressed, donor-specific antibodies do not develop. Because regulation is targeted rather than global, the rest of the immune repertoire remains intact. (Xing P, Cold Spring Harb Perspect Biol, 2012)
Figure 1. Central and peripheral tolerance, from Boucault, et al. Frontiers in Transplantology. InTech, 2016.
How to engineer tolerance?
Two ingredients are essential:
Durable donor hematopoiesis- the ongoing antigen supply. Donor CD 34+ progenitors seed myeloid and lymphoid lineages, creating APCs that shape both thymic education and peripheral presentation. (Müller AM, et al. Blood, 2014)
A temporary regulatory shield- the “quiet room” that keeps effectors in check, while deletion consolidates. This can be built with lymphodepletion/irradiation, or with biological tools such as infused regulatory T cells, costimulation blockade, and cytokine control. (Ohm B, et al. Semin Immunopathol, 2025)
The duration of chimerism determines how deep the deletion runs. The withdrawal of immunosuppression is the test that shows whether the repertoire has been permanently rewritten.
Two complementary experiments that prove the rule
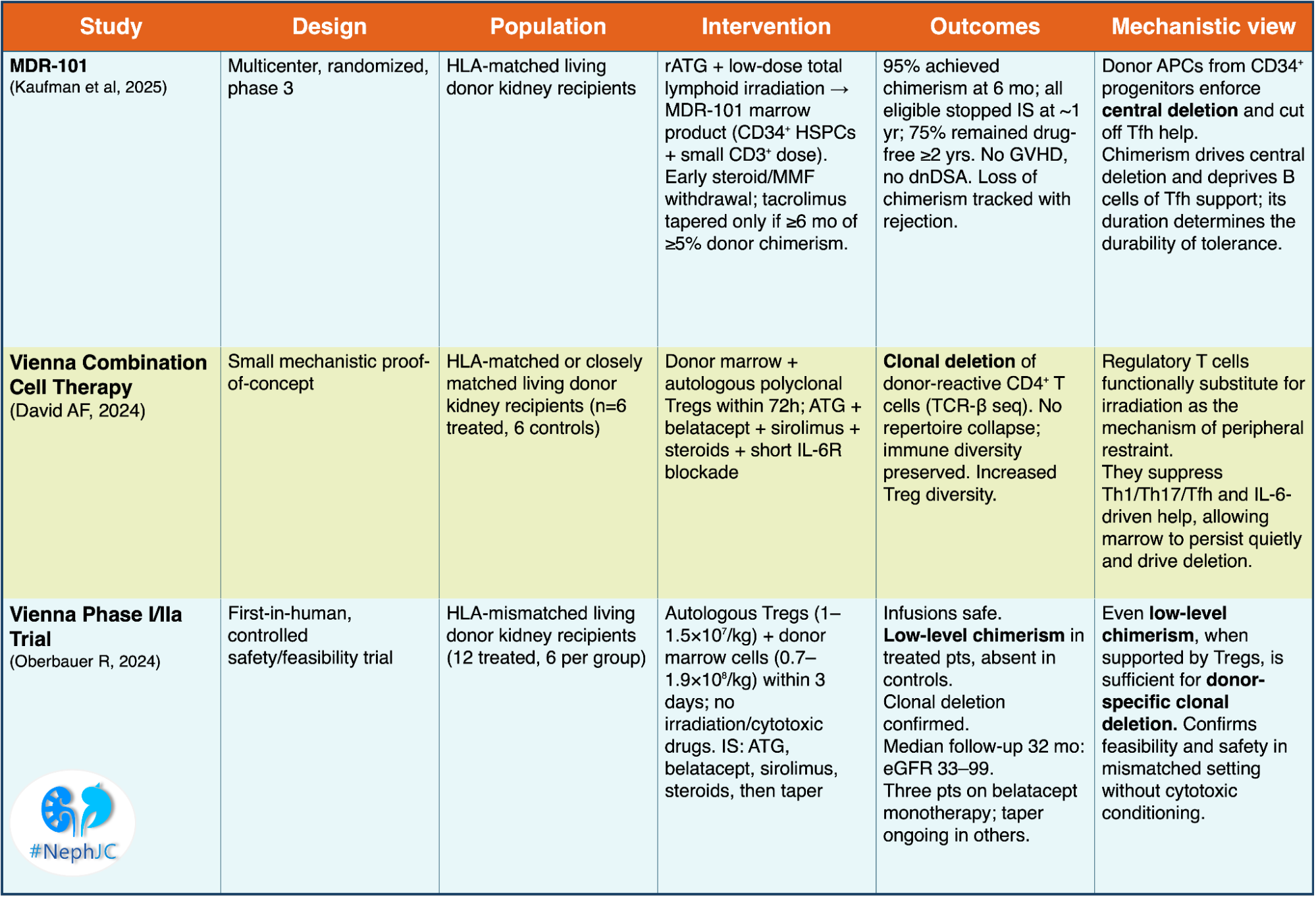
1) MDR-101 mixed-chimerism 2:1 randomized trial (Kaufman et al, Am J Transplant, 2025)
Design: 20 HLA-matched kidney recipients received rATG, low-dose lymphoid radiation, then donor marrow (MDR-101) with CD 34+ progenitors and a small T-cell fraction. Steroids were stopped by day 10, MMF by day 39; tacrolimus was tapered only if chimerism ≥5% persisted for 6 months, with full withdrawal at 12 months.
Results: 95% achieved mixed chimerism at 6 months. All eligible patients stopped immunosuppression at 1 year; 75% remained immunosuppression-free beyond 2 years. No graft versus host disease (GVHD), no dsDNA. Loss of chimerism correlated with rejection, while stable chimerism predicted tolerance.
Summarizing, donor APCs persist in driving central deletion and cut off follicular T helper support for B cells. rATG+ total lymphoid irradiation enables this process. Chimerism is the mechanistic currency that buys deletion; time on chimerism is the interest that makes it last.
2.A. Vienna combination cell therapy (David AF, et al, EBioMedicine. 2024) - focused on direct mechanistic proof of tolerance induction
It was a small trial in HLA-matched or closely matched living donor kidney transplantation. The intervention was donor bone marrow plus autologous regulatory T cells given within 72 hours. Induction was done with anti-thymocyte globulin, belatacept, sirolimus, steroids, and short Il-6 receptor blockade.
Key findings:
Clonal deletion of donor-reactive CD4+T cells demonstrated by T-cell receptor beta sequencing.
No collapse of immune repertoire; global immunity preserved;
increased diversity within the regulatory T-cell compartment.
In summary, regulatory T cells can replace irradiation as the regulatory shield, allowing donor marrow to persist long enough to drive deletion and tolerance mechanisms
2.B. Vienna phase I/IIa trial (Oberbauer R, et al, JASN, 2024) proof-of-concept in the clinic
It’s the first-in-human expansion trial in HLA-mismatched living donor kidney transplantation. The intervention was autologous regulatory T cells plus donor marrow cells infused within 3 days. No irradiation or cytotoxic conditioning was used. Immunosuppression: ATG, belatacept, sirolimus, steroids, followed by taper.
Key findings:
Treated patients developed low-level donor chimerism, absent in controls
Clonal deletion of donor-specific T cells, confirmed by sequencing
Stable kidney function at 32 months (eGFR 33-99 ml/min/1.73 m2)
Three patients successfully reduced belatacept monotherapy.
This study demonstrated that the approach is safe and clinically feasible in mismatched transplants. Even low-level of chimerism, when supported by regulatory T cells, are sufficient to induce donor-specific clonal deletion and permit drug minimization.
How do these pieces connect?
The underlying biology is consistent across approaches; the difference lies in the tools. MDR-101 establishes early immune restraint with ATG plus total lymphoid irradiation, whereas the Vienna protocols achieve the same effect through regulatory T-cell infusion combined with interleukin 6 receptor blockade. In both settings, donor CD 34+-derived chimerism provides sustained antigenic presentation, enabling clonal detection of donor-reactive T cells. When chimerism is stable, patients don’t develop donor-specific antibodies.
Thresholds are important: MDR-101 requires ≥5% donor leukociyte chimerism for ≥6 months before IS withdrawal. Persistence of chimerism predicted tolerance, whereas its loss was associated with immune reactivation and rejection in a subset of patients.
The quality of immunity matters more than the quality of immunosuppression: Vienna’s sequencing data demonstrated selective loss of donor-reactive clonotypes without global repertoire collapse, while MDR-101 showed that when deletion is sufficiently deep, long-term IS-free survival can be achieved without GVHD or dsDNA.
Broader context
Circling back to the inception of this post, think of tolerance as needing two levels: (1) donor-derived antigen over time (through mixed chimerism) and (2) the regulatory shield that keeps effectors quite long enough for deletion to set in.
Different strategies are ways to build that regulatory shield:
Costimulation blockade (eg: CD28-B7 or CD40-CD154) stops native T cells from getting their “go” signal and deprives B cells of Tfh help. On its own, it usually isn’t enough, but it synergizes with chimerism of Tregs. (Wekerle T, et al, J Exp Med, 1998| Schoenbrunn A, et al, J Immunol, 2012)
T reg-based therapies (polyclonal, donor-enriched, or CAR-Tregs) extend the Vienna concept: impose selective regulation while central selection accumulates. Their survival is best when supported with low dose IL-2 or mTOR inhibitors. (Lamarche C, Kidney Int Rep, 2022)
Tolerogenic dendritic or macrophage cells act as “teacher” cells, presenting donor antigen in a non-inflammatory context that nudges T cells into anergy or a regulatory phenotype. (Lin J, et al, Front Immunol, 2021)
Humoral modulation (IL-6R blockade, BAFF inhibition, selective B-cell targeting) quiets the germinal response, buying time for deletion to consolidate and reducing the risk of donor-specific antibodies. (Oberbauer R, Front Med, 2021| Ohm B, et al. Semin Immunopathol, 2025)
Commentary by Cristina Popa