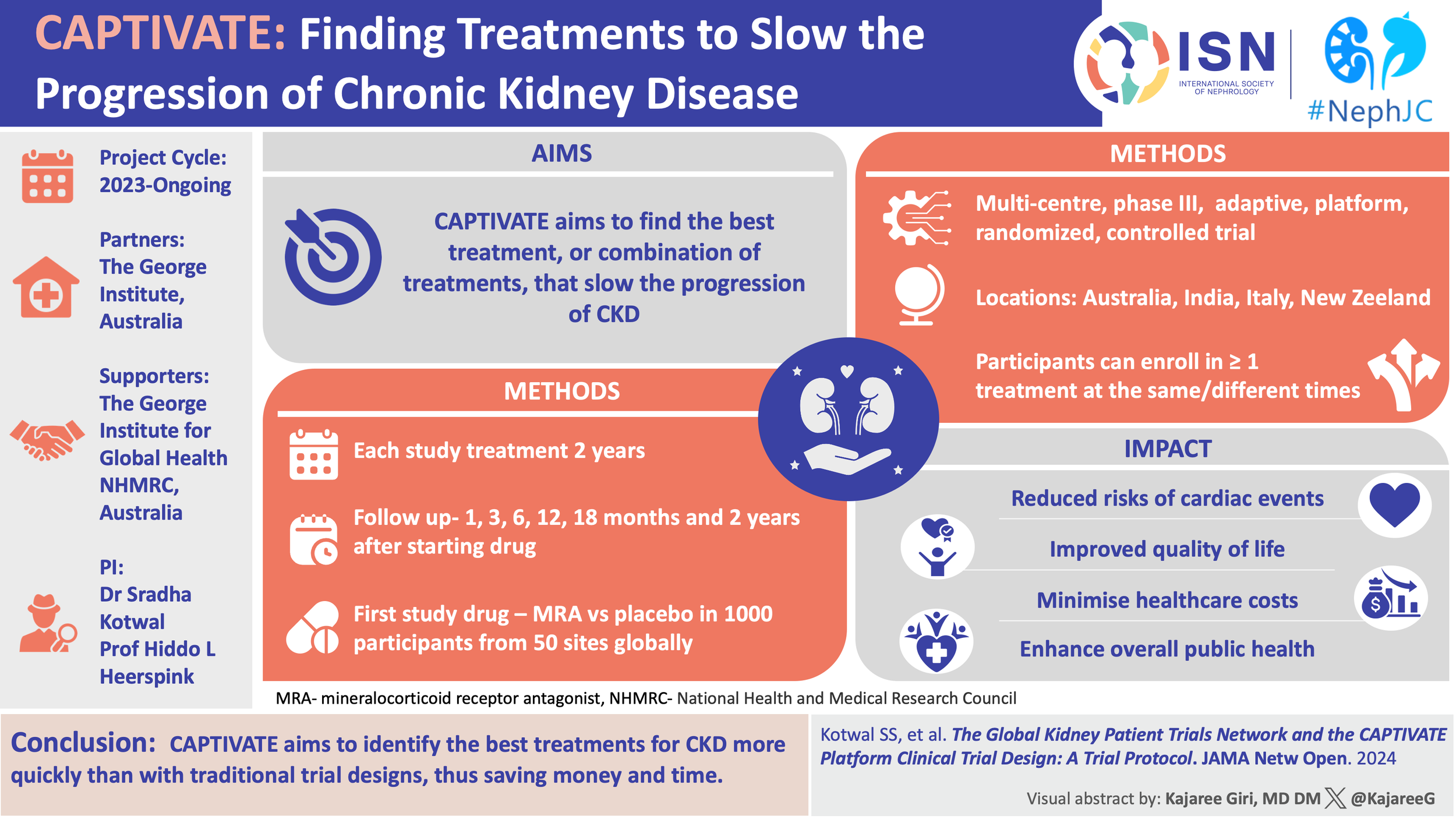
A #NephTrials chat
Tuesday, November 21
9 PM Eastern, 3 AM Central European, 7:30 AM Indian Standard Time
#NephTrials is an ongoing initiative between #NephJC and the ISN-ACT group. The ISN-ACT (Advancing Clinical Trials) is an International Society of Nephrology (ISN) initiative to encourage existing infrastructures within ISN to improve the global nephrology community’s participation in clinical trial research.
Introduction
In such a few but poetic words, Anitha @Ren_familia has described Calciphylaxis so aptly as “a venomous art” and a “malefic condition”. It almost always is a debilitating condition with ischemic and/or necrotic skin lesions seen predominantly in end-stage kidney disease patients receiving dialysis but can also be seen in advanced CKD. The “darkness” of this disease comes from the fact that there are, to date, no high-quality studies to guide the optimal treatment approach for patients with calciphylaxis. Our current treatment approach is based upon data from observational studies and clinical experience with multidisciplinary management, including dermatologists, wound care specialists, nephrologists, and pain and palliative care support.
Why is it so difficult to conduct RCTs (randomized controlled trials) evaluating treatments for calciphylaxis?
Calciphylaxis has a low incidence- incidence rate of 1-4% percent per year among patients receiving maintenance hemodialysis.
Due to its devastating prognosis, there is a lack of tolerance for prolonged placebo treatment and
Low tolerance for single active treatments in the face of no treatment response.
There has been a requirement for a biopsy-based diagnosis of calciphylaxis in current RCTs.
The treatment of the disease typically involves multiple modalities of clinical care; and there is a lack of standardized methodology for assessing calciphylaxis outcomes in RCTs.
The Better Evidence And Translation for Calciphylaxis (BEAT-Calci) trial has been developed to specifically address these barriers.
What's the evidence on treatment of calciphylaxis so far, and what can we expect from BEAT-Calci?
Well, the evidence so far is mostly all anecdotal!
Although 3 randomized clinical trials of sodium thiosulfate have been attempted for calciphylaxis including CALISTA, no results of these studies have been reported or published. But sodium thiosulfate is commonly used to treat calciphylaxis. Bisphosphonates have been proposed to offer benefit for patients with calciphylaxis. A prospective series of 11 patients with calciphylaxis on dialysis found that the addition of bisphosphonates slowed calciphylaxis progression in all patients 2-4 weeks after starting treatment, and improved outcomes when used with supportive care. Vitamin K may also play a role in the treatment of calciphylaxis. Vitamin K deficiency is implicated in calciphylaxis via reducing gamma-carboxylation of matrix Gla protein (MGP)- a calcification inhibitor. In Vit-K CUA, a phase 2 RCT of 26 patients with calciphylaxis, oral phytonadione reduced uncarboxylated MGP. Results were just published as late-breaking poster in Kidney Week this month and demonstrated that phytonadione therapy led to improvements in pain intensity and total lesion surface area in a higher number of patients compared with placebo. The effect of phytonadione was seen even in the absence of application of cinacalcet and intralesional sodium thiosulfate. A larger phase 3 study is now planned to establish the efficacy and safety of this agent in calciphylaxis.
Increased clearance may improve calciphylaxis, given that hyperphosphatemia and hyperparathyroidism improve when kidney function improves. Baldwin et al reported a case series of 7 patients at a single institution in whom a systematic multi-interventional treatment strategy consisting of trigger-agent cessation (calcium-based phosphate binders, alphacalcidol, and warfarin), wound management, and antibiotic therapy, supplemented by intensified hemodialysis (4 hours daily for 7 days followed by 5-6 times weekly), intravenous sodium thiosulfate (12.5-25 g intravenously 3 times a week), and attempted oxygen therapy (given through a face mask or hyperbaric chamber as tolerated by patient circumstance). All 7 patients identified with biopsy-proven calcific uremic arteriolopathy were treated with this regimen with 6 of 7 showing complete recovery. See below for the commonly used therapies across the world and the variation given the lack of a proven management strategy.
Figure courtesy of the BEAT-Calci research study team
Above mentioned therapies remain unproven, with no controlled studies to support these approaches. As Dr. Berry says in his viewpoint article on Platform trials- “one population, one drug, one disease” strategy with conventional or traditional RCT designs is not always successful in finding effective therapies for many diseases. Adaptive platform design such as used in BEAT Calci can accelerate efforts to identify effective treatments, especially combination treatments and treatments tailored to particular subgroups of patients, for challenging diseases.
In a previous #NephTrials edition we did a deep dive on master protocols.
Master protocols allow a framework in which a centralized screening platform and a common protocol format allows multiple parallel studies. Master protocols may involve one or more interventions in multiple diseases or a single disease with multiple interventions, each targeting a particular biomarker-defined population or disease subtype. Basket, umbrella, and platform trials are all types of master protocols. Multiple substudies under an over-arching master protocol are designed with an aim to achieve better coordination. This can be done by sharing key design elements as well as study materials such as patient data and removing redundancies of stand alone traditional trials each requiring their own infrastructure.
Table from Heerspink et al, Diabetes Obes Metab. 2018
What is a platform trial?
Platform trials, also referred to as multi-arm, multi-stage (MAMS) design trials, are trials that evaluate several interventions against a common control group and can be perpetual. Trial learning is also perpetual as interventions enter and exit while conclusive evidence is being generated.
Master protocols are established to specify study population, key enrollment features, outcome measures based on which multiple interventions can be studied.
Bayesian decision rules are used to determine if therapies with low probabilities of success or side effects should be discontinued and therapies with high probabilities of future success should advance for further study.
Figure from Park et al The Lancet Global Health Vol 9 May 2021
What are adaptive trial designs?
Adaptive trial designs allow modification of an ongoing trial in response to the accumulating data and evidence to increase statistical efficiency or to achieve better participant outcomes.
Examples of Adaptive trial designs:
Figure from Pallman et al Feb 2018
Adaptive designs add a review–adapt loop to the linear design–conduct–analysis sequence.
Figure from Pallman et al Feb 2018
Perry Wilson and Matt Sparks wrote about adaptive trials for Nephmadness 2016.
What are Adaptive Platform designs?
Fixed trial designs are conventional 2-arm trial designs in which we have a predetermined sample size and analyze the data once at the end.
The focus of an adaptive platform trial is a disease or condition rather than a particular intervention. The “adaptive” nature of the platform uses information generated during trial conduct to alter subsequent randomization and operational decisions in a pre-specified way.
Figure from Nature Reviews Drug Discovery Aug 2019
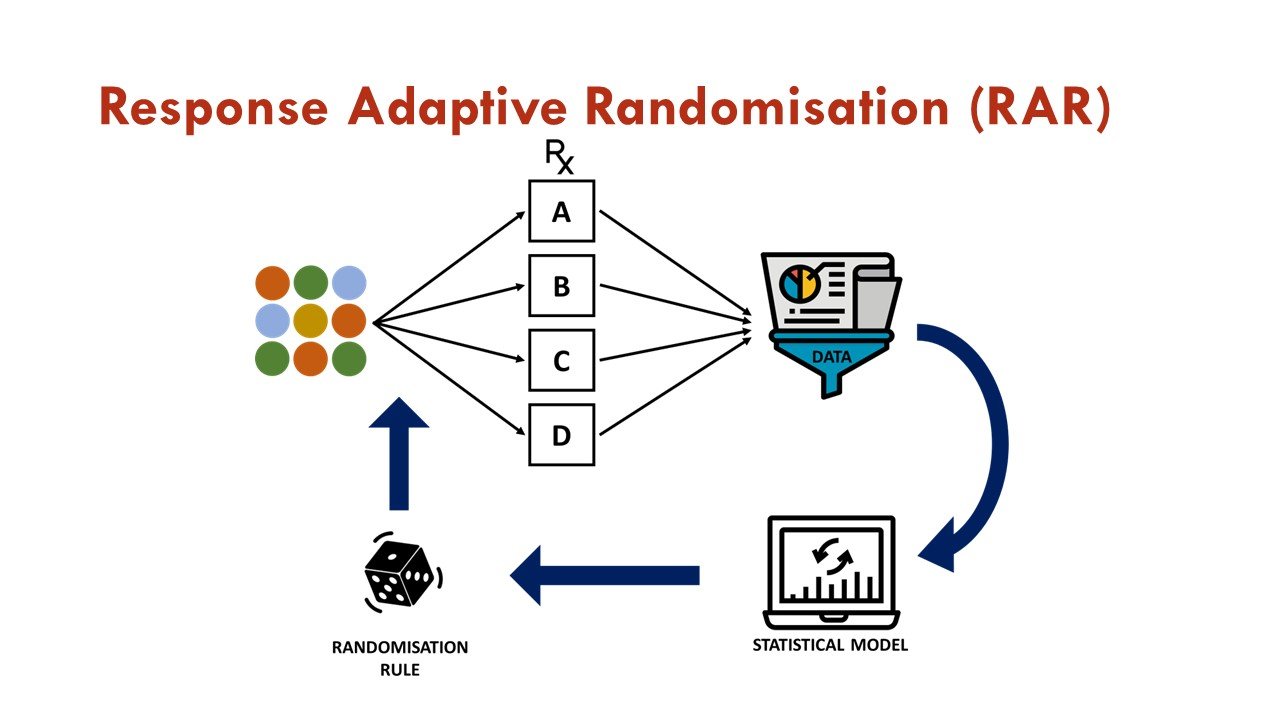
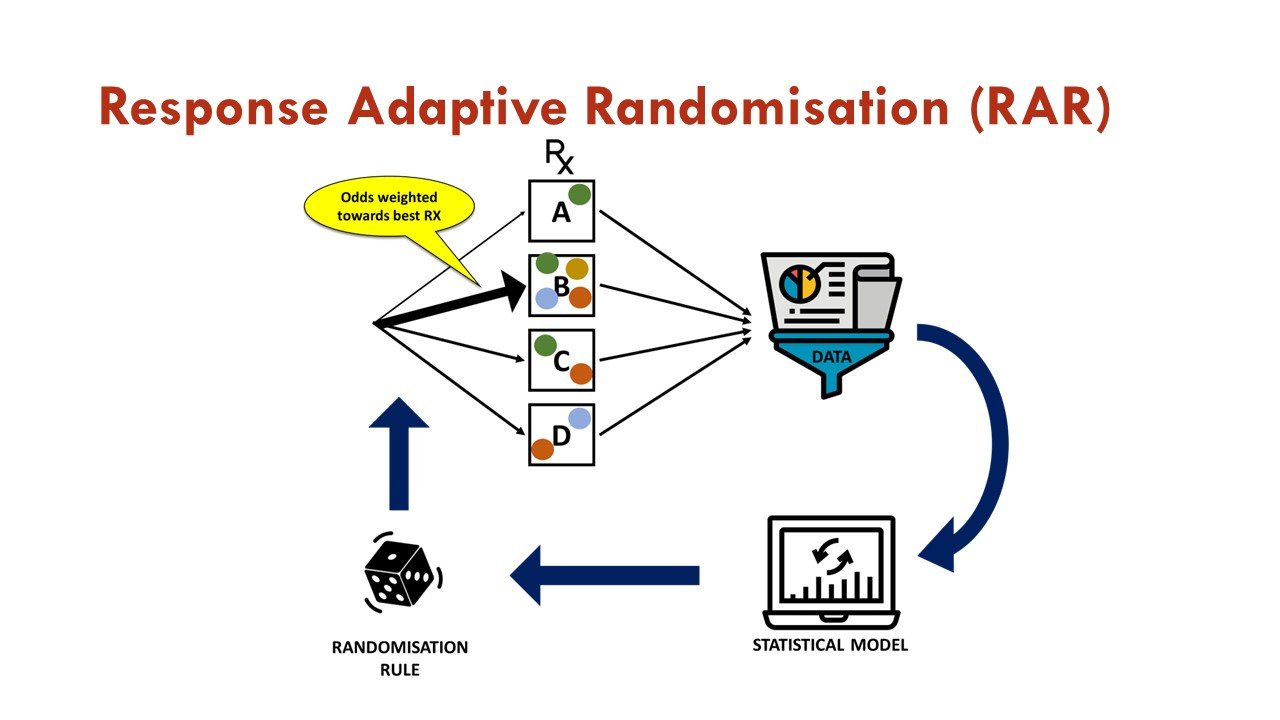
What is response adaptive randomization (RAR)?
RAR is one of the commonly used adaptations in platform trials, in which accumulating data is used to change the assignment of additional participants as the trial progresses. In essence, after initial 1:1 randomization against a common control arm, subsequent randomization weights are associated with estimates of treatment effects generated from previously enrolled patients via a pre-specified algorithm. This allows more patients to be assigned to the more promising treatment arm, increases overall trial efficiency, and is an attractive feature for trial participants.
Figures courtesy of the BEAT-Calci research study team
What are some of the important differences between traditional trials and platform trials?
Advantages of Adaptive Platform trial designs:
Allow rapid and efficient knowledge generation, especially if embedded in routine clinical practice, can drive continuous improvements. This can help in narrowing the translation gap between evidence generation and clinical care.
Common platform and infrastructure allow efficient use of control arms as well as available financial resources.
Allow for frequent learning by implementing pre-planned interim analyses that use prespecified algorithms to guide decisions.
Standardized operating procedures and master protocols implemented across different institutions reduce interference due to geographical variability and can allow the trial to be conducted in multiple sites, including low-income countries. For example, RECOVERY started in the United Kingdom and has now expanded to sites across Asia and Africa.
A heterogeneous population varying in biomarkers, tumor genetics, illness severity, disease risk factors, or age can be studied under one trial to find the best treatment for each of these subgroups.
Disadvantages
Adaptive platform trials, by nature, are complex in design, analysis, and logistics of conduct.
Significant pretrial time expenditure to define the master protocols, patient selection and stratification, trial adaptations, and statistical modeling is required.
Bias occurs when randomization ratios between trial groups are allowed to change over time, when some sites that have different mortality rates restrict randomization to a specific subset of trial agents, when some sites do not allow randomization to the control group, or when administered concomitant treatments are different between trial treatment groups.
In a platform trial, it may be difficult or burdensome to blind patients to different arms due to different modes of administration or dosing.
Platform trials: past, present, future
The first ever and oldest platform trial which started in 2005 (18 years in running!) is STAMPEDE evaluating therapies for men with advanced prostate cancer. Since 2005, almost 12,000 participants have joined the trial. When the trial opened in 2005, there were five "original comparisons" and ten interventions have entered this platform trial thus far. Several agents have graduated from phase II evaluation with seamless transitions into phase III evaluations. The ground-breaking I-SPY 2 trial of neoadjuvant treatment for locally advanced breast cancer started in 2010 and is still ongoing, and continues to be a major influence on the development of innovative trial designs in oncology and beyond.
In a recent study, Park et al. (2019) identified 16 platform trials initiated between 2001 and 2019, with numbers increasing over time. Now compare this to Vanderbeek et al (Jan 2022) who reported that 58 COVID-19 platform trials were globally registered between January 2020 and May 2021. 16 trials have added new therapies.
COVID-19 pandemic essentially forced us to conduct clinical trials which are more patient-centric than ever before. The pandemic provided a unique research environment for which platform designs were particularly well-suited for rapid drug development and testing. In addition to the RECOVERY trial, REMAP-CAP which has been running since 2016, was able to quickly expand from community-acquired pneumonia to also COVID-19. Within 3 months of starting the RECOVERY trial, due to its efficient adaptive platform design, we knew that dexamethasone, an inexpensive and widely available drug, decreased deaths in severely ill patients with COVID-19 infection. Under conventional phase 1 to phase 3 clinical trial infrastructure, it can take up to 15 years to bring a drug to patients who desperately need it.
It’s a glowing example of how, if done well, experimenting with the experiment itself can accelerate drug development by a decade.
The time is ripe for the uptake of transformative changes in clinical trial designs in nephrology! In July 2016, the International Society of Nephrology hosted a CKD summit of more than 85 people with diverse expertise and professional backgrounds from around the globe. The purpose was to identify and prioritize key activities for the next 5–10 years in clinical care, research, and advocacy and to create an action plan and performance framework. The group proposed that 30% of patients with CKD should be involved in relevant clinical trials by 2030. The ambition is big, and the recent move towards innovative trial designs such as registry based RCTs and adaptive designs is indeed a huge leap forward for our field.
The BEAT Calci Trial
Better Evidence and Translation for Calciphylaxis (BEAT-Calci) is a global randomized, adaptive, multi-center, platform trial that will evaluate multiple interventions across several domains of therapeutic care.
Visual Abstract of the BEAT-Calci trial design from Divya Bajpai and the ISN Social Media team
Here is a video on Adaptive RCTs and the official website for BEAT-Calci.
The objective of the study is to establish high-quality evidence on the effect of a range of interventions on the BEAT-Calci Wound Assessment Scale (BCWAS) in patients with kidney failure and newly diagnosed calciphylaxis. BCWAS, a score that incorporates clinically significant calciphylaxis events, will be used to determine each participant's outcome.
Main Platform Inclusion Criteria:
Age ≥ 18 years
Currently receiving hemodialysis, or peritoneal dialysis that can be converted to hemodialysis, with planned ongoing hemodialysis a minimum of three times per week for at least the duration of the protocolized calciphylaxis treatments within this trial
Have a new calciphylaxis ulcer present for less than 10 weeks
Eligible for randomization in at least one recruiting domain
The participant and treating physician are willing and able to perform trial procedures
Platform Exclusion Criteria:
None
Active follow-up is for 26 weeks, and passive follow-up is for 4.5 years.
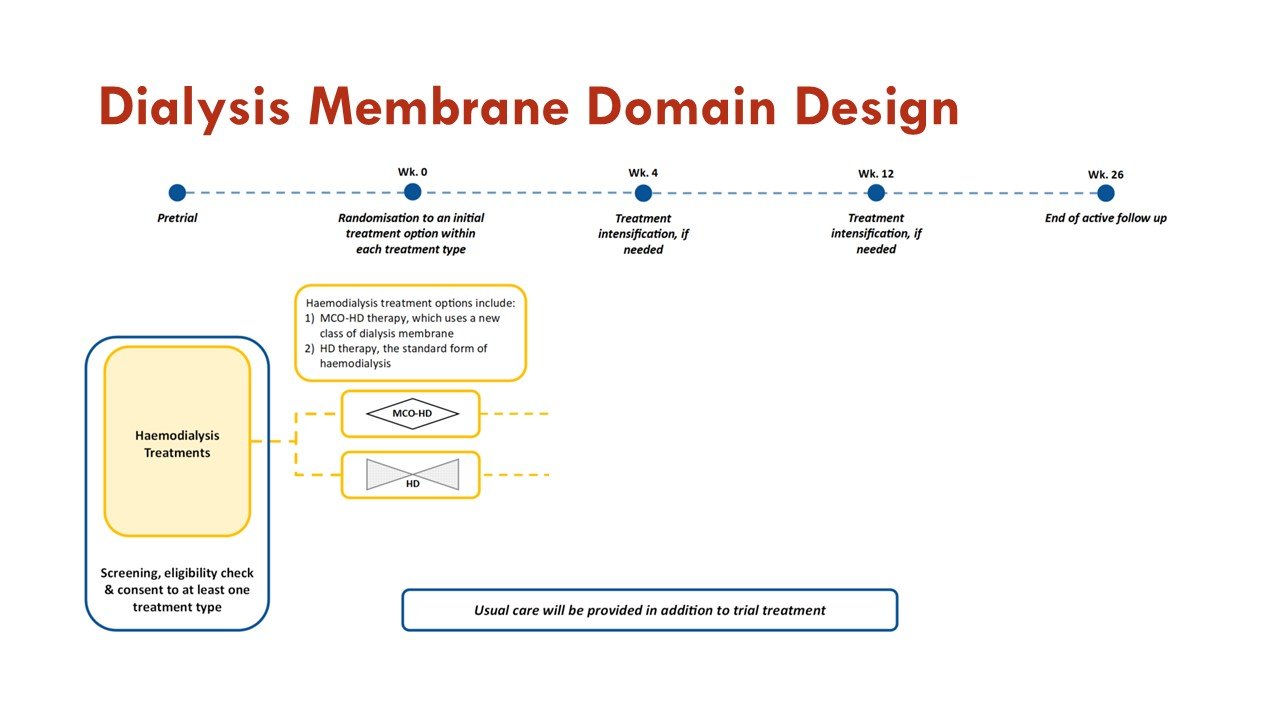
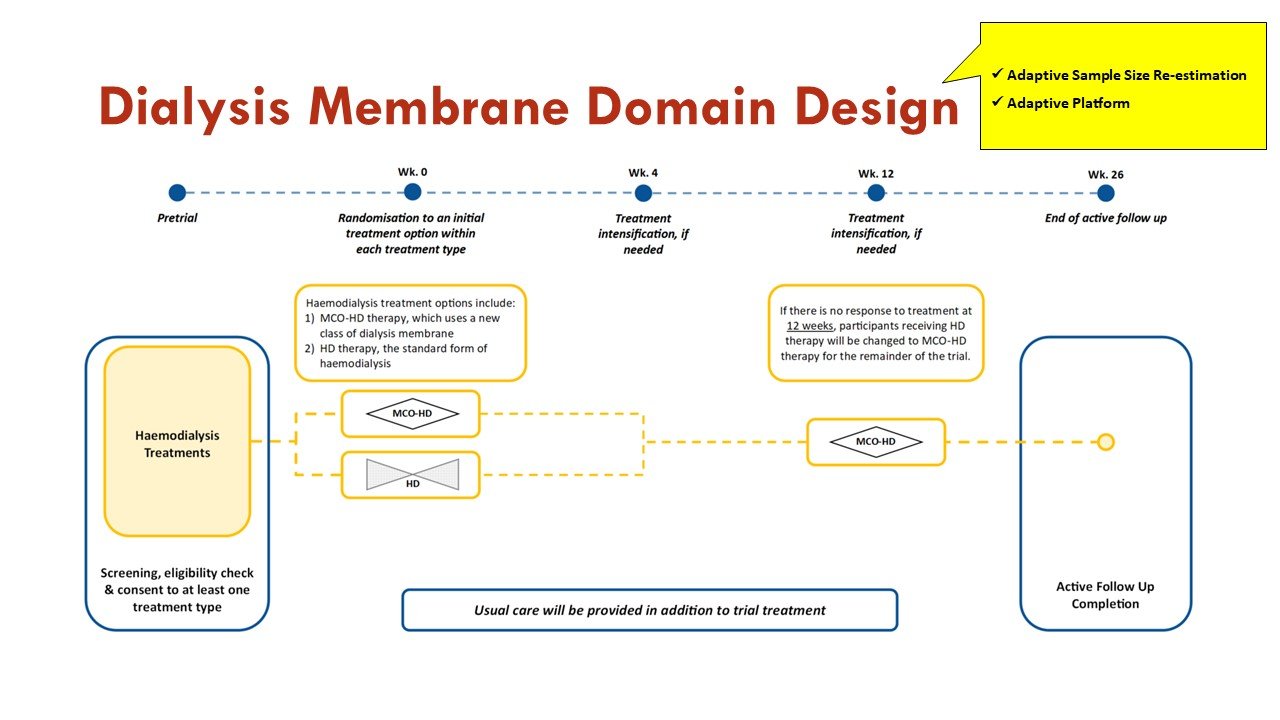
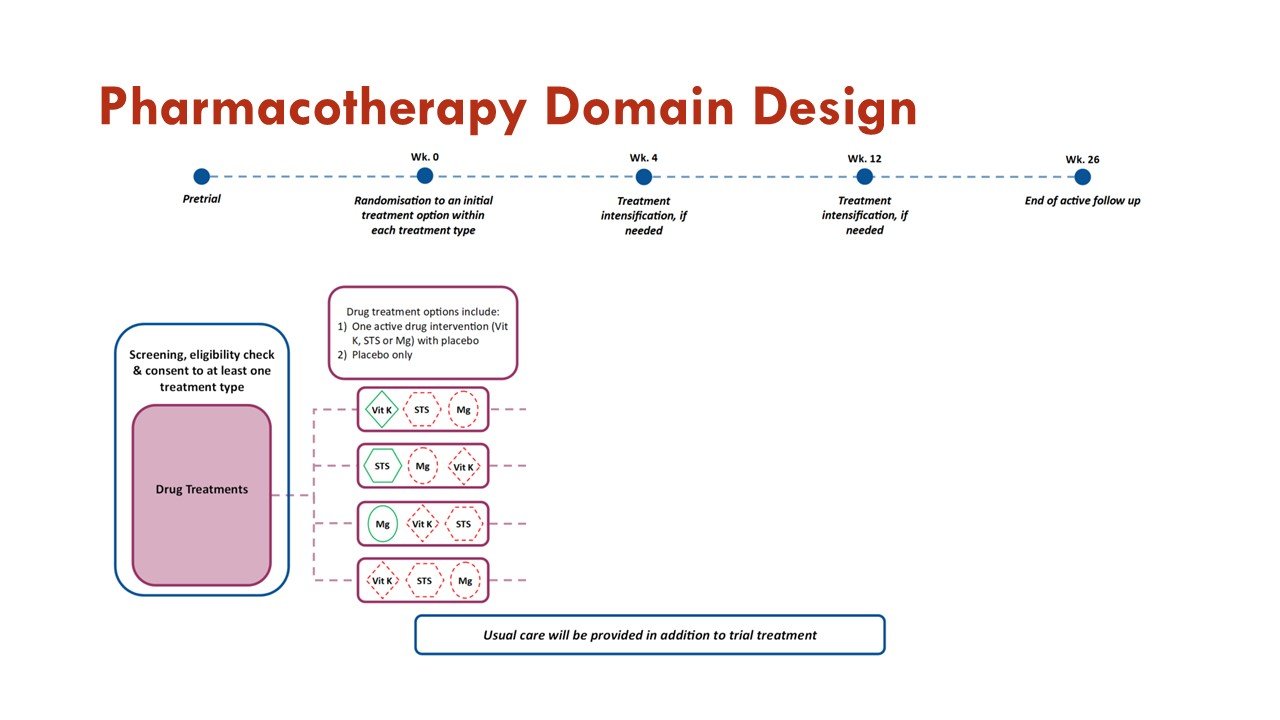
The trial has a Dialysis Membrane Domain and a Pharmacotherapy Domain.
What are the adaptations to the Study Design?
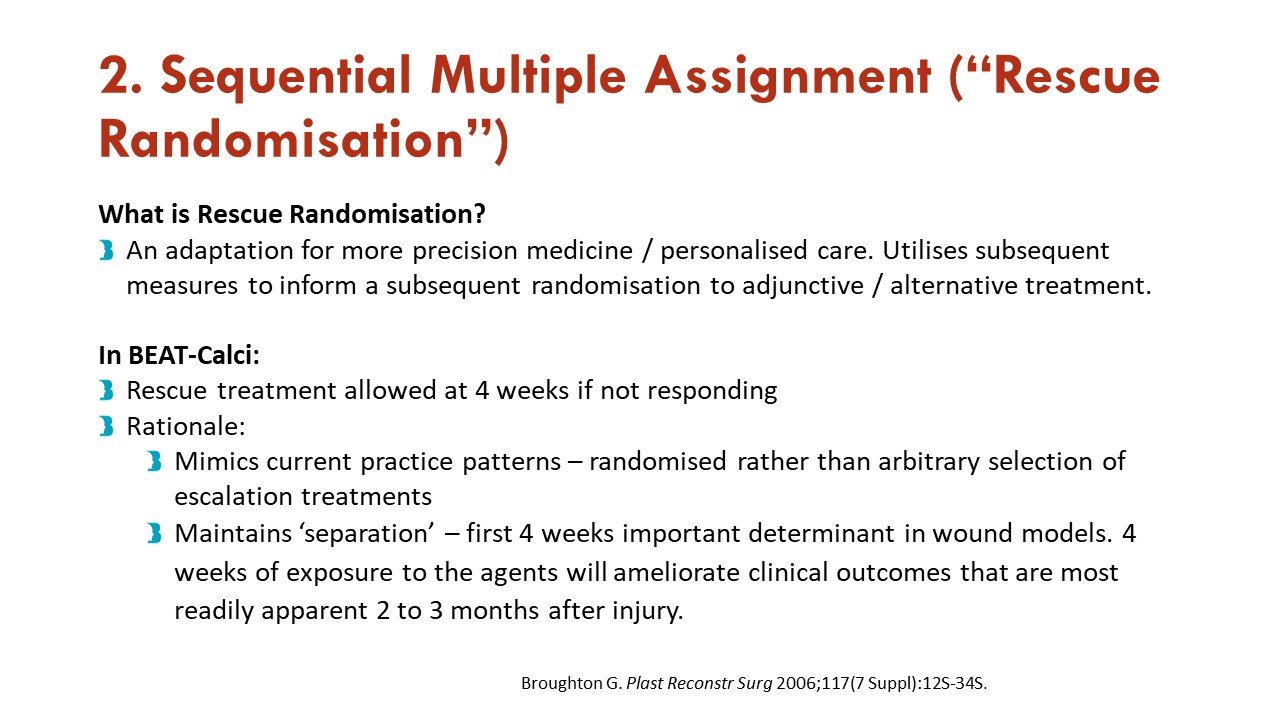
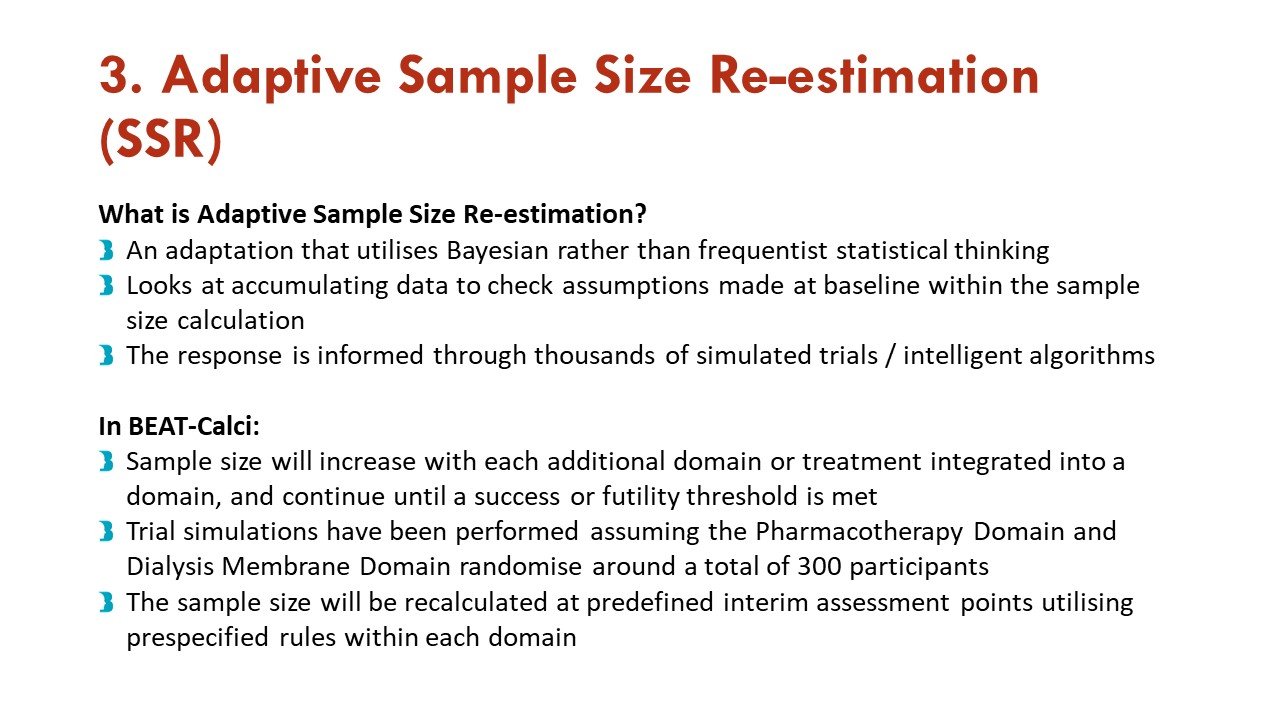
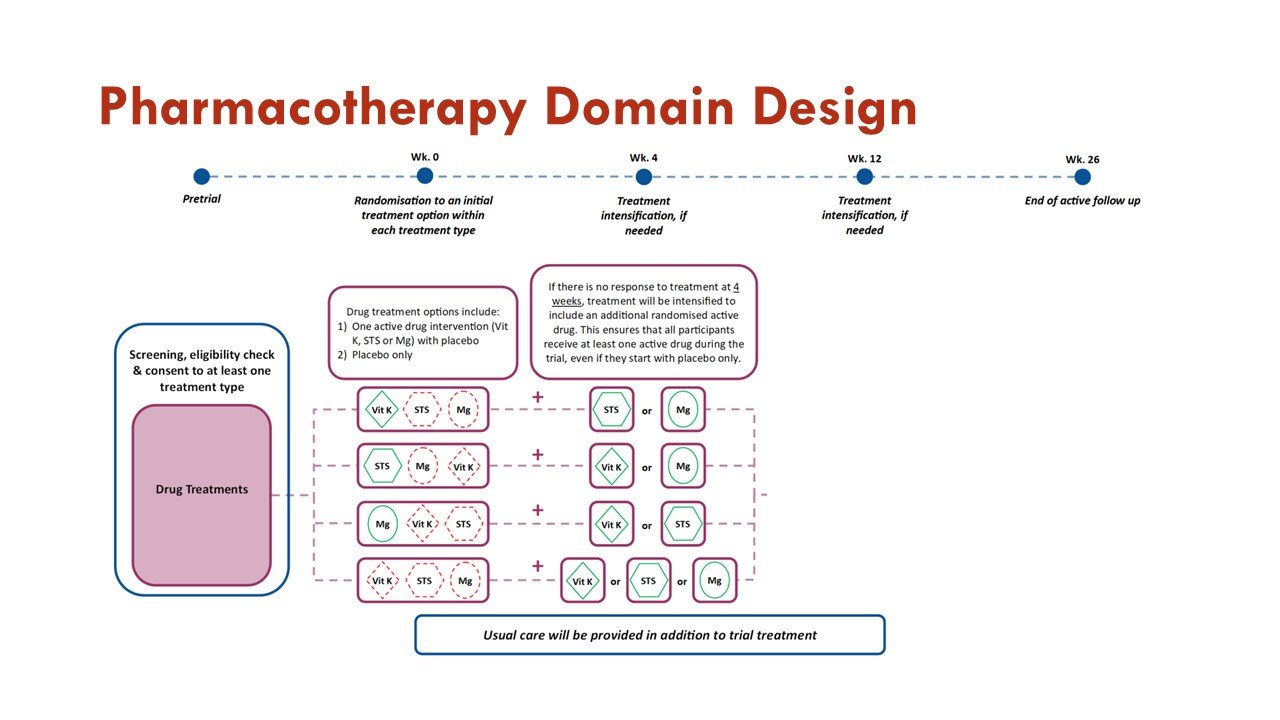
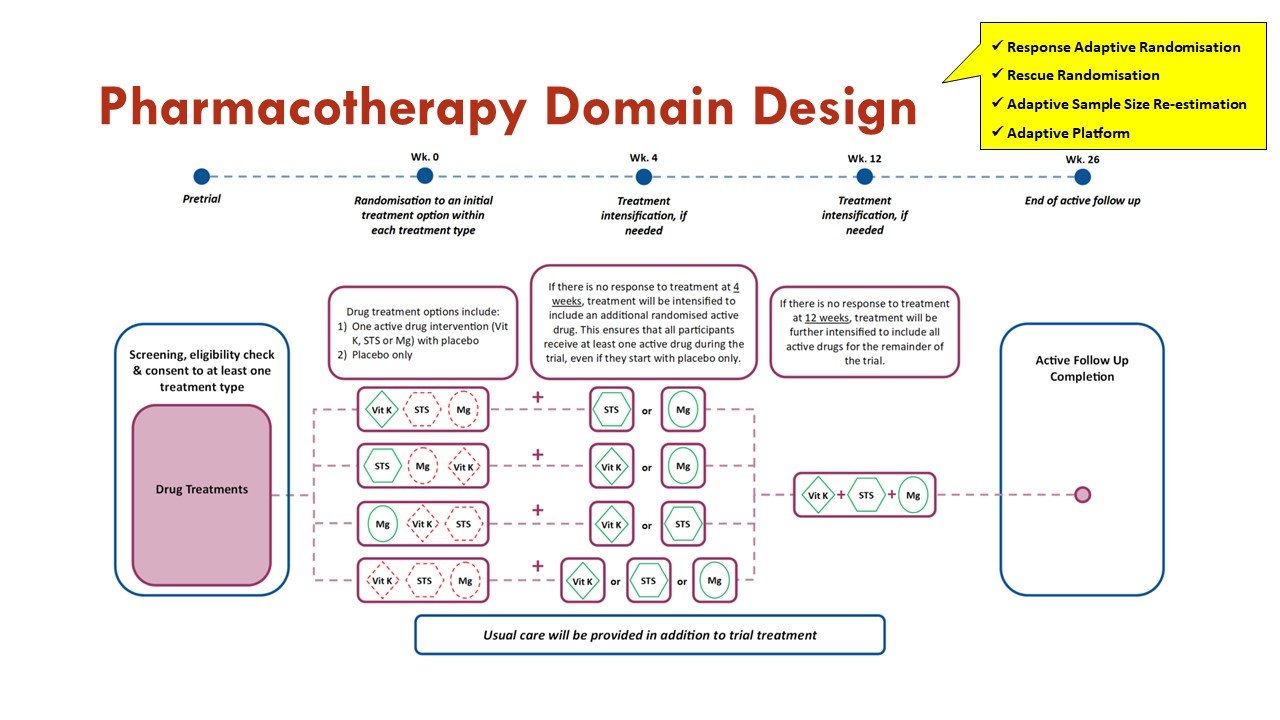
Apart from the response adaptive randomization (mentioned above), this trial also incorporates sequential multiple assignment (allowing rescue if no improvement at 4 weeks - important given calciphylaxis natural history) and adaptive sample size re-estimation. See more on that in the figures:
Slides courtesy of the BEAT-Calci research study team
As an adaptive platform trial, additional domains of care, and additional interventions within existing domains, will be introduced into the platform as they become scientifically and operationally feasible.
Slide courtesy of the BEAT-Calci research study team
Recruitment of participants into BEAT-Calci will continue until prespecified statistical rules are met. Participants will be recruited from a number of countries, commencing with Australia, New Zealand, and the United Kingdom.
What are the main interventions and outcomes of the BEAT-Calci trial?
The primary objective of the Dialysis Membrane Domain is to investigate whether treatment with medium cut-off hemodialysis is superior to high flux hemodialysis in improving outcomes measured on the BEAT-Calci Wound Assessment Scale, in patients with kidney failure and newly diagnosed calciphylaxis.
High-flux hemodialysis (HD) is the standard dialysis therapy used in centers worldwide. In this domain, treatment with HD is the control arm.
Medium cut-off hemodialysis (MCO HD) uses the medium cut-off (MCO) dialyzer, which has a pore size between those of a standard high-flux dialyzer and a high cut-off dialyzer. MCO HD therapy allows clearance and subsequent reduction of larger middle molecules, which may play a crucial role in stopping or limiting the inflammatory and calcification processes in calciphylaxis. In this domain, treatment with MCO HD is the intervention arm.
Slides courtesy the BEAT-Calci research study team
The primary objective of the Pharmacotherapy Domain is to investigate whether treatment with any of the active interventions within the domain are superior to placebo in improving outcomes measured on the BEAT-Calci Wound Assessment Scale, in patients with kidney failure and newly diagnosed calciphylaxis.
Within this domain, sodium thiosulfate injections, vitamin K1 capsules, and magnesium citrate tablets will be compared to each other and to matched placebo.
Slides courtesy the BEAT-Calci research study team
Primary outcome measure is to determine whether the addition of the intervention changes the sentinel ulcer from baseline to week 12 on the BEAT-Calci Wound Assessment Scale. This is an 8-point ordinal categorical scale of change since baseline, which will be used to determine each participant's outcome.
Slide courtesy BEAT-Calci research study team
Despite not having effective and proven therapies till date the future looks promising with the ongoing BEAT Calci and other clinical trials such as Calciphyx- phase 3 RCT presented at ASN’s Kidney Week 2023 studying SNF472, and RHEO-CAL which is an RCT to compare the efficacy of rheopheresis as adjuvant treatment.
Like Margaret Thatcher said “You may have to fight a battle more than once to win it.”
And the renaissance in clinical trials in nephrology is indeed a beacon of hope for us to win this battle against Calciphylaxis.