#NephJC Chat
Tuesday Apr 14 9 pm Eastern
Wednesday Apr 15 9 pm IST
Wednesday Apr 15 9 pm BST
Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China
Hua Su, Ming Yang, Cheng Wan1 Li-Xia Yi, Fang Tang, Hong-Yan Zhu, Fan Yi, Hai-Chun Yang, Agnes B. Fogo, Xiu Nie
PMID: pending Full Text at Kidney International
Introduction
SARS-CoV2 infection, a beta-coronavirus of bat origin, is responsible for the current pandemic of COVID-19 disease. The majority of individuals have infection localized to the respiratory tract; however, 15% have systemic disease with viremia (Huang et al, Lancet 2020). Acute kidney injury (AKI) has been reported in 5-15% of patients (or higher at 15-39% for critically ill patients) and is associated with higher mortality in infected individuals (Naicker et al, Kidney Int 2020), resulting in 60-90% mortality (compared to an overall mortality rate of 0.3 to 10%). The risk of mortality increased with the stage of AKI (Cheng et al, Kidney Int 2020). Sixty-three percent of patients with AKI develop proteinuria, of which 34% is nephrotic range. Hematuria has also been reported in 26-44% of individuals (Cheng et al, Kidney Int 2020). Kidney injury may be due to direct infection through ACE2 receptors on tubular epithelial cells or through a systemic inflammatory response to the virus (Naicker et al, Kidney Int 2020). A systemic inflammatory response syndrome is characterized by high levels of IL-2, IL-7, IL-10, G-CSF, MCP1, MIP-1α, and TNFα and is associated with an increased risk of intensive care unit (ICU) admission and poor clinical outcomes (Naicker et al, Kidney Int 2020). Co-morbid conditions may also increase the risk of poor outcomes, including hypertension, diabetes mellitus, chronic kidney disease (CKD), obesity, cardiovascular disease, and pregnancy, though good adjusted data is still awaited. While AKI and CKD increase the risk of morbidity and mortality, the precise cause of AKI is largely unknown. In this study, the authors examined the kidney pathology of 26 decedents with COVID-19 to determine the histopathologic findings.
Clinical and laboratory parameters of COVID-19 autopsies:
A series of 26 autopsies with COVID-19 confirmed by positive SARS-CoV2 molecular testing by RNA PCR was performed in China. These cases were all rapid autopsies with a post-mortem interval of 6 or fewer hours, which reduces autolytic artifacts within tissue. In this series, all patients presented with respiratory distress, requiring intensive care unit admission and died of respiratory failure with acute respiratory distress syndrome. The patients were older (age range 39-87, with a mean of 69) and most had medical comorbidities, including cancer (n=6, 23%), hypertension (n=11, 48%), and diabetes mellitus (n= 3, 12%). There were more males (19) than females (7). All of these risk factors for death have previously been identified (Chen et al, Kidney Int 2020).
Approximately one-third of the decedents had kidney dysfunction prior to death (9 of 26, 35%). Factors contributing to kidney injury in COVID-19 patients included hypoxia, abnormal coagulation, and rhabdomyolysis. Only 9 of the patients had a pre-morbid urinalysis. Seven of nine had proteinuria and 6 of 9 had hematuria. Two patients had acute pyelonephritis. A summary of the clinical and laboratory parameters of the COVID-19 patients during hospitalization are provided below and in Table 1.
Table 1 from Su et al, Kidney Int 2020
A summary of the treatments provided during hospitalization to these patients is provided in Table 2. Continuous renal replacement therapy was required in 5 patients (19%). Only 4 (15%) patients had exposure to nephrotoxic drugs. Antiviral therapy was given to 16 (62%) patients. Steroid therapy was provided to 16 (62%) patients. The clinical treatments of this cohort are similar to what has been described in others, including fluid support to maintain hemodynamic stability and continuous renal replacement in individuals with acute renal failure. The efficacy of antivirals is uncertain and is currently being assessed in clinical trials (NCT04276688, NCT04261907, NCT04273763, NCT04280705, NCT04336904, among others). New recommendations by the World Health Organization indicate that steroids are not beneficial in COVID-19 infection and are contraindicated, due to prolonging viremia (Russell et al, Lancet 2020).
Table 2 from Su et al, Kidney Int 2020
Kidney pathology of COVID-19 autopsies:
All cases in the series showed mild to severe acute tubular injury (figure 1a and b). Acute tubular injury was characterized by a loss of the proximal tubular brush borders, vacuolar degeneration (non-isometric in most cases), coagulative necrosis (4 cases), hemosiderin granules within tubular cytoplasm, and pigmented casts within tubular lumens.
Two cases showed evidence of acute pyelonephritis, with neutrophil-rich interstitial inflammation, bacterial colonies within kidney parenchyma (figure 1c), and abscess formation (figure 1d). There was no significant interstitial inflammation (restricted to only areas of interstitial fibrosis and tubular atrophy) in the other 24 autopsies.
Within proximal tubular epithelium, hemosiderin deposition was present within four cases (figure 1e) and pigmented casts were seen within tubular lumens (figure 1f). Hemosiderosis can be seen within hemolytic states. The presence of hemosiderin deposition and pigmented casts within the same case are concerning for hemoglobin casts. Other possibilities include myoglobin casts or cellular debris casts, and rhabdomyolysis has been reported to occur in some patients with COVID-19 infection (Jin et al, Emerging Infect Dis 2020).
There was evidence of glomerular ischemia, with three of the cases show fibrin thrombi within the glomerular capillary loops (figure 1 g) and presence of contraction of the glomerular capillary tufts with exudation of plasma within Bowman’s space in seen in 7 cases (figure 1 h). This was rarely associated with an overlying epithelial cell proliferation (pseudocrescent formation). No proliferative changes were identified within glomeruli, such as endocapillary hypercellularity or true crescents (cellular or fibrocellular).
Since red blood cell fragmentation and platelet thrombi were not identified, it is possible that segmental fibrin thrombi within glomerular capillary loops could be indicative of innocent thrombi usually seen within a systemic hypercoagulable state, which may be seen in renal vein thrombosis. Other studies have suggested that COVID-19 patients have an increased risk of coagulation (Zhou et al, Lancet 2020), and several patients within this cohort had a positive D-dimer level during hospitalization, consistent with hypercoagulability (Table 1).
Peritubular capillary congestion was identified within COVID-19 decedents, but is a relatively common finding in autopsy kidneys. The red blood cell aggregates (highlighted below with glycophorin A/ CD235 immunohistochemistry, figure 3a) in peritubular capillaries had negative CD61 (platelet marker) staining, a finding suggestive of the absence of platelet thrombi. CD31, an endothelial cell marker, highlights the peritubular capillaries.
Immunofluorescence (performed on the paraffin-embedded tissue) did not show any significant findings, except for a single case of IgA nephropathy. Given that IgA nephropathy is relatively common within Asian populations (2-5% based on autopsy studies), this was likely an incidental finding.
Ultrastructural analysis by electron microscopy revealed presence of virions, consistent with direct infection of kidney parenchyma, and subendothelial electron-lucent widening suggestive of endothelial injury. This is displayed below, and in figure 2. Figures 2a-d, viral particles, showing uniformity and presence of spikes; figure 2e, subendothelial electron lucent widening; figure 2f, congestion of peritubular capillaries by red blood cells. Virions were characterized by being 65-136 nm in size, with 20-25 nm spikes and a solar ‘corona’ configuration. The presence of subendothelial lucency was consistent with endothelial activation. Fibrin tactoids or platelet aggregates, as seen in thrombotic microangiopathy, were not observed in any COVID-19 cases.
The receptor for SARS-CoV2, ACE2, was overexpressed within kidney parenchyma in COVID-19 kidney sections (figure 3 c), but was also present in high levels in tubular epithelium of a control (figure 3 b). While restricted to proximal convoluted tubules normally, Bowman’s capsule and visceral epithelial cells were showed positivity in the COVID-19 patients shown.
Positive immunofluorescence staining for SARS-CoV2 antibody within tubular epithelial cells was also present within 3 of 6 stained cases (figure 3 d). Notably, this was the same antibody showing staining within kidney parenchyma by Diao et al, however, in our laboratory, we found staining with this same antibody to be non-specific within staining of tubular nuclei in non-COVID-19 patients (Larsen et al, Kidney International Reports 2020).
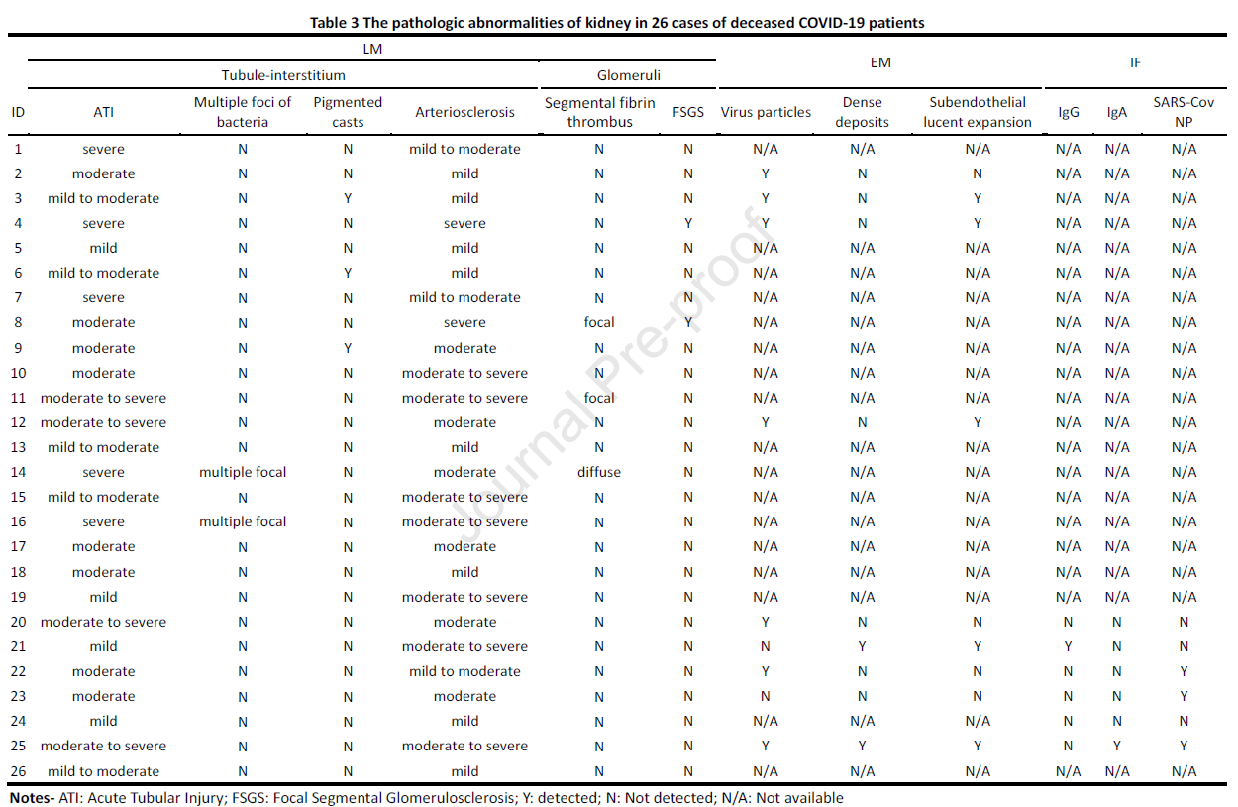
A summary of the majority of the pathologic abnormalities found within kidney sections of COVID-19 autopsies is provided in Table 3. All cases shown acute tubular injury (ATI), 9 of which were severe. Two cases showed pyelonephritis with multiple foci of bacteria and an inflammatory reaction. Three cases showed pigmented casts within tubular lumens, which can occur as a result of cell debris, myoglobin secondary to rhabdomyolysis, or hemoglobin due to systemic hemolysis. Hypertensive-related vascular changes were seen in all decedents, and was moderate to severe in 18 patients. Seven decedents had viral particles identified ultrastructurally within proximal convoluted tubules, distal tubules, or within podocyte cytoplasm. While red blood cell fragmentation and platelet rich thrombi were not identified in any cases, some had focal microangiopathic changes, including fibrin thrombi in glomerular capillaries in 3 and subendothelial electron lucent widening in 5. Immune complex deposition was not significant, except for a single case of IgA nephropathy.
Table 3 from Su et al, Kidney Int 2020
One limitation of this study is that not all of the decedents studied had evidence of kidney failure at the time of death. In fact, the cause of death of all patients was acute respiratory distress syndrome, while only 7 of 26 had evidence of multiorgan system dysfunction at the time of death. Another autopsy study, also within a Chinese cohort, also shown severe acute tubular injury in 6 of 6 decedents, similar to this study (Diao et al, MedRxIV 2020).
Acute tubular injury may be multifactorial. This could be due to prerenal injury, systemic inflammatory response syndrome, rhabdomyolysis, direct cytopathic effect of viral invasion of ACE2-expressing renal tubular epithelial cells (+/- visceral and parietal epithelial cells within glomeruli), or drug-induced toxicity (Fortarezza et al, Journal of Nephropathology 2020).
Given that a Chinese population may be relatively ethnically homogenous, it is possible that we may be missing pathology that may be enriched within other populations. In a recent case report by Larsen et al (Kidney International Reports 2020), an African American COVID-19 patient with two risk alleles for APOL1 had collapsing glomerulopathy. It is possible that SARS-CoV-2 infection could serve as a “second hit” to APOL1 nephropathy, as seen within HIV and parvovirus B19 infections. In the figure below from this case report, acute tubular injury with epithelial simplifcation, reactive nuclear changes and mitotic figures is shown (Figure 1a), collapsing glomerulopathy (Figure 1b) with severe podocyte foot process effacement (Figure 1c), and presence of subendothelial tubuloreticular inclusions (Figure 1d). The presence of tubuloreticular inclusions, although seen in multiple disease states, is consistent with an increase in type I interferons. It is possible that SARS-CoV-2 infection, through interaction of toll-like receptors and activation of plasmacytoid dendritic cells, produces a systemic inflammatory response syndrome through release of high levels of type I interferon. This could serve as a second hit in APOL1 nephropathy in susceptible individuals.
Fig 1 from Larsen et al, KI Reports, 2020
Interestingly, in this case, in situ hybridization for SARS-CoV2 nucleoprotein failed to detect the presence of viral RNA within tissue, despite good total RNA integrity with maintenance of a high stringency positive control (PP1B), figure 2, not shown. No viral particles were identified within podocytes by electron microscopy.
In conclusion, kidney pathology findings in COVID-19 disease include:
Acute tubular injury and/or necrosis in all cases
Glomerular ischemia and endothelial injury
Peritubular capillary congestion and fibrin thrombi, may represent a systemic hypercoagulable state
Ultrastructural evidence of virions
Evidence of co-morbid pathologies – diabetic glomerulopathy and/or arterionephrosclerosis
Collapsing glomerulopathy, possible exacerbation of APOL1 nephropathy
Summary by Tiffany Caza,
Pathologist, Arkana Laboratories, Little Rock, AR
NSMC Faculty