Kidney Int. 2021 Jun;99(6):1478-1486
doi: 10.1016/j.kint.2021.02.027
A Randomized Control Trial to Investigate the Effects of Intra-dialytic Cycling on Left Ventricular Mass
PMID: 34023029
Cost-Effectiveness Analysis: A Cost-Effective Analysis of the CYCLE-HD Randomized Controlled Trial. Daniel S. March et al, KI Reports 2021
“Hearts will never be practical until they can be made unbreakable”
“Exercise is a celebration of what your body can do”
Introduction
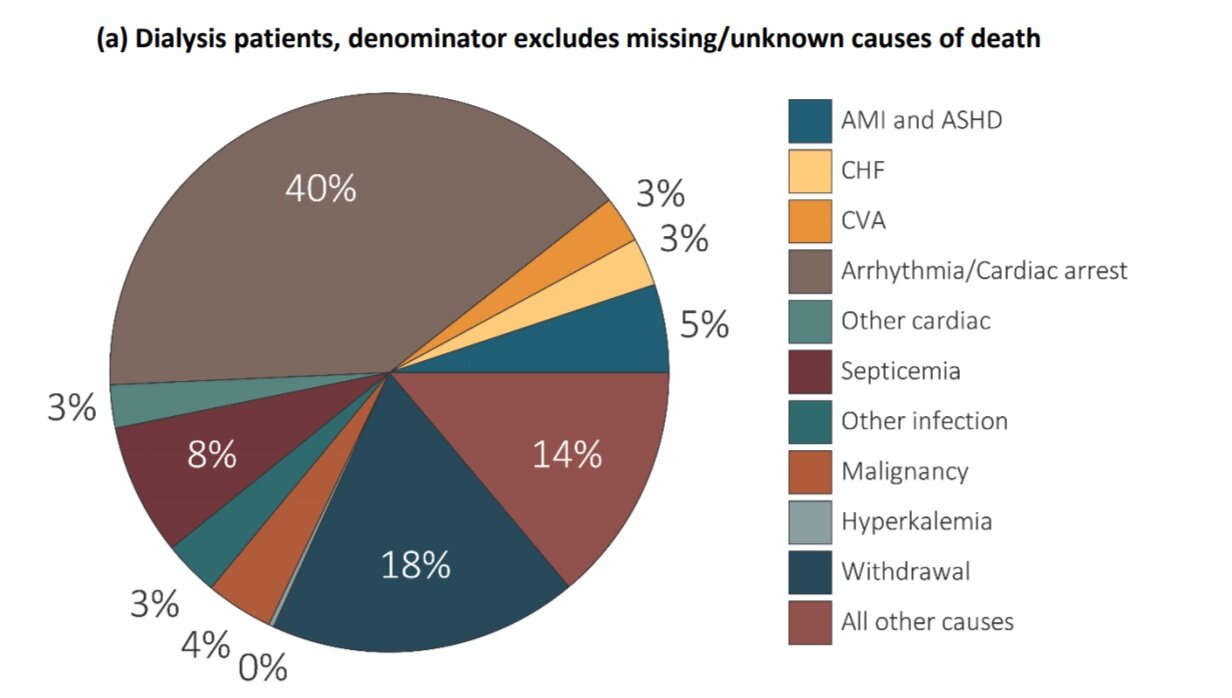
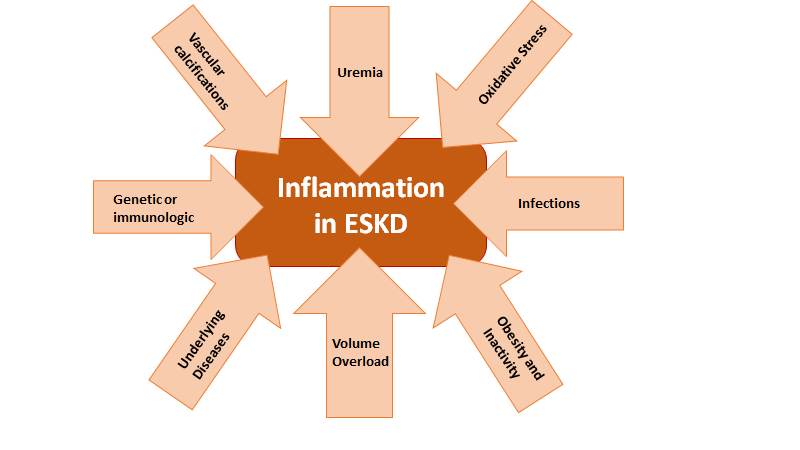
According to the WHO, ischemic heart disease is the number one attributable cause of death worldwide. Cardiovascular disease (CVD) accounted for approximately 9 million or 16% of all deaths in 2019. CVD is also a significant cause of morbidity and mortality among patients with ESKD. Patients on maintenance dialysis have a disproportionately high CVD mortality, with more than twice the rate seen in non-ESKD patients (Figure below from USRDS, 2015). Nephrologists recognize many CVD-related hazards in ESKD are unique to this patient population. Traditional risk factors are unable to explain the excess CVD burden founding ESKD and therapeutics that are proven in non-ESKD patients often fall short in patients with ESKD. Additionally, ESKD patients have many physiologic conditions that create a high inflammatory milieu, which may exacerbate CVD.
USRDS Unadjusted Percentages of Deaths in Dialysis Patients in 2015 (denominator excludes patients with unknown causes of death)
Systemic inflammation leads to pathologic modifications in the heart’s function and structure. These changes may include remodeling of the ventricles, increased arterial stiffness and myocardial fibrosis (Aoki J, et al. Kidney Int, 2005). Left ventricular hypertrophy (LVH) is found in up to 90% of ESKD patients after only 2 years on dialysis, and the degree of LVH is strongly and independently associated with cardiovascular events (Zoccali C, et al. Kidney Int, 2004).
Exercise improves CVD outcomes across a number of different chronic diseases. There is biological plausibility that exercise may have anti-inflammatory effects as well (Lavie CJ. Circ Res, 2015).
Physicians are aware that there are a number of barriers and benefits which patients consider before adopting a regular exercise regimen. Patients may choose to exercise for a multitude of reasons besides decreased CVD mortality. In a survey of ESKD patients many reported the desire for better strength, energy, independence and sleep. ESKD patients also reported they were less likely to perform regular exercise due to pain, shortness of breath, fatigue, and time constraints (Moorman D, et al. Clin J Am Soc Nephrol, 2019).
Prior studies have suggested that intradialytic exercise improved Kt/V, peak oxygen consumption, intradialytic hypotension, physical performance and quality of life when performed consistently over several months. A meta-analysis of previous studies did not show any significant musculoskeletal or cardiovascular complications between intradialytic exercise groups and control groups (Sheng K, et al.Am J Nephrol, 2014). The current study, CYCLE-HD, looked at the effects of a 6-month intradialytic cycling (IDC) exercise program on left ventricular (LV) mass.
Etiologies of Inflammation in ESKD
The Study
Design
The CYCLE-HD was a prospective, cluster-randomized, open label blinded study that took place in 3 hemodialysis units in the United Kingdom. The days when patients were dialysed (i.e. MWF or TTS) were randomized - so they overall had 6 clusters from the 3 units. However, individual patients within those clusters could decide to participate/consent, though the allocation was concealed until after the enrolment/consent process was done. For more on cluster RCTs, check out this NephTrials post from Manasi Bapat.
Intervention
Patients were enrolled in a 6-month progressive IDC program. Cycling took place on calibrated cycle ergometers (Letto series; Motomed, Reck, Germany).
Patients performed IDC three times a week during dialysis with a goal of 30 minutes of continuous cycling. Patients were monitored for a rate of perceived exertion (RPE). A goal RPE was 12-14, with adjustment of cycle resistance to obtain goal effort. Progressive training was allowed to assist participants in completing the goal of 30 minutes of continuous cycling. A 1-month run-in was performed to allow patients to get used to the machines.
Study Population
This was a very pragmatic study design, and all adult patients on hemodialysis for more than 3 months were eligible.
Measures
All patients underwent Cardiac Magnetic Resonance (CMR) imaging on a 3-T platform. Scans were done on non-dialysis days 18-24 hours after most recent dialysis treatment. Scans were read by a single blinded reader. LV mass and volumes, aortic pulse-wave velocity and native T1 mapping were performed.
Secondary outcomes data was also obtained. 48-hour Holter monitoring was done at the start and conclusion of the study. Readings were interpreted by a single blinded observer for frequency of PVCs and complex ventricular tachycardia. Physical function tests were performed at the start and end of the study. Participants were evaluated on an Endurance Shuttle Walk and Short Physical Performance Battery tests. Accelerometers measured total steps and average metabolic equivalents of tasks over days worn. Blood sampling for Troponin I and NT-proBNP as well as pre- and intra-dialytic blood pressures, as well as weights were recorded.
Outcomes
The primary end point:
Change in the LV mass compared to baseline at 6 months.
Secondary end points included:
Cardiovascular structure and function
Physical activity and quality of life.
Number of IDC sessions completed over time.
Change in the amount of power (RPE over cycling time) achieved.
Statistics
The sample size was ascertained from two prior studies. The study was designed to detect a 15 gram difference in LV mass between the intervention and the control groups after 6-months of exercise. A planned sample size of 130 patients provided 80% power to detect a 15 g difference in LV mass. Analyses were done with an intention to treat; the primary outcome was analyzed using linear mixed effect regression models.
Funding
The research was funded by NIHR in the United Kingdom and supported by Kidney Research UK. There was no input from NIHR for design, data collection, data analysis and interpretation.
Economic analysis
A cost-effectiveness study (March et al, KI Reports 2021) was conducted in conjunction with CYCLE-HD study.
Results
The trial was conducted from March 2015 through April 2018 with a total of 155 participants, of whom 130 completed the CMR scans (65 in each group). During the process 14 were lost to follow up (due to death, withdrawal, transplantation, hospitalization, or change in treatment modalities). 101 patients completed the trial protocol with 50 controls and 51 intervention patients. See figure 1 below.
Figure 1: Study Consort Design, from Graham-Brown et al, KI 2021
Patient characteristics
Baseline demographics are provided in table 1 (as they should be). 73% were male sex. About 12% of study population had ischemic heart disease.
Table 1: Baseline characteristics of the study population, from Graham-Brown et al, KI 2021
In the IDC training group, 3137 of 4376 (71.7%) exercises sessions were completed. Over the course of the study there was a decrease in IDC exercises, 77% in the first month compared to 61% in the sixth month. The reasons for the decrease in exercise sessions included: patient declined (30%), patient felt unwell (21%), and pain (14%).
The exercise intensity was able to be maintained from the beginning (RPE 12.4 +/- 2.0 for the first month compared to 12.7 +/- 1.9 for the last month). Though the duration of exercise declined, the power increased over the study period. See Supplementary Figure 1.
Supplementary Figure 1: Details of exercise intervention delivered over the study period. A. Percentage of sessions attempted per month over study period; B. Changes in rating of perceived exertion over study period; C. Exercise session duration over study period and; D. Average power expended per month throughout the study.
Primary endpoint
There was a statistically significant reduction in LV mass in the intervention group. IDC resulted in a 11.1 gram decrease in LV mass (95% CI, -15.79 to -6.43 g; P < 0.0001).
Figure 2 from Graham-Brown et al, KI 2021, showing change in LV mass
Secondary endpoints
The table below summarizes the secondary endpoints. Though the quality of life p values are > 0.05, there is an interesting difference in the EQ5D scale.
Table 2 from Graham-Brown et al, KI 2021
Adverse Events
There were 51 adverse effects noted with 37 in the IDC group versus 14 in the control group. However, none of them were believed to be related to the intervention. The serious adverse effects in both groups involved cardiovascular and dialysis access. The table below displays the adverse effects.
There were 9 total deaths, 5 of which occurred prior to baseline assessment being completed. Of the remaining, one IDC death was due to CVD and 2 due to nonfatal cerebrovascular events (one control and one IDC patient).
Discussion
Strengths
The study was able to show a reduction in LV mass over a 6-month period. Use of cardiac MR rather than echocardiography makes the data more reliable. Additionally, other markers of CVD including aortic stiffness and potentially myocardial fibrosis also improved. The dropout rate and serious adverse effects were acceptable. The trial however was unable to show a statistical difference in physical function or quality of life.
Limitations
The IDC intervention and control groups both had attrition rates higher than the 10% anticipated, but equal to similar studies.
Other factors such as anemia, volume status, blood pressure have an effect on LV mass. The nature of the RCT ameliorates much of this, however, adjustment for baseline covariates could potentially help, which could not be performed given the small sample size. Note the difference in blood pressure, and need for erythropoietin and iron as potentially important covariates.
There is a lack of generalizability given study size, high refusal to participate rate and the fact that the dialysis centers were all in the same clinical network.
Study designers conclude that they were unable to quantify the exact amount of exercise that is needed to decrease the LV size.
As the authors mention with the supplement, due to lack of systematic documentation, there may have been incomplete recording of adverse events.
Change in LV mass is a surrogate outcome. How much of this would actually translate into change in clinical outcomes is uncertain. Though LV mass is usually considered a valid surrogate, in CKD, this association is somewhat imprecise (Badve et al, AJKD 2016).
Is 6 months follow up sufficient? As is seen, there was a decline in sessions where participants were willing to exercise - would these keep dwindling even further with longer followup?
Conclusion
There appears to be benefit to patients participating in an intra-dialytic exercise program. Reduction in LV mass, and by association possibly CVD burden, by IDC is a cost effective way to add quality adjusted life years to hemodialysis patients. Exercise promotes overall better cardiovascular health in comparison to expectant management. There is still much work needed to understand the best ways to quantify the effect of exercise, the minimum effort needed to have an impact on patients, and the overall effect on patient mortality.
Summary Prepared by
Brian Rifkin
Interventional/General Nephrology
Hattiesburg Clinic
Hattiesburg, MS
Nephrology Fellow at McGovern Medical School
Houston, TX
NSMC Interns, Class of 2021