#NephJC Chat
Tuesday August 8 at 9 pm Eastern
Wednesday August 9 at 8 pm BST, 12 noon Pacific
J Am Soc Nephrol. 2017 Apr 11. pii: ASN.2016111179. doi: 10.1681/ASN.2016111179. [Epub ahead of print]
Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis.
Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, Burst V, Grundmann F, Jadoul M, Szombati I, Tesař V, Segelmark M, Potarca A, Schall TJ, Bekker P; CLEAR Study Group.
PMID: 28400446
Introduction
The ANCA-associated vasculitides (AAV) are a group of uncommon, systemic diseases well known to nephrologists and rheumatologists. Presentation of AAV varies from asymptomatic abnormal lab results to organ failure and death.
Over the past 25 odd years the vasculitis community has taken a structured approach to improving the lives of patients with AAV:
Standardizing disease assessment and classification
Using these standards to perform randomised controlled trials examining both induction and maintenance treatment regimens
Using database studies to quantify long-term risks of disease and treatment
A recent key finding is that some of the morbidity and mortality risk of AAV may result from the treatments used rather than the disease itself. This knowledge is driving attempts to streamline treatment regimens to avoid unnecessary harm, with a major focus on reducing exposure to glucocorticoids (GC). It is this that motivated the randomised trial of the C5a receptor inhibitor, Avacopan (Ava), that we are discussing this week.
The Study
What did they do?
32 treating centres funded by ChemoCentryx (who make the drug and paid all authors either fees or as employees)
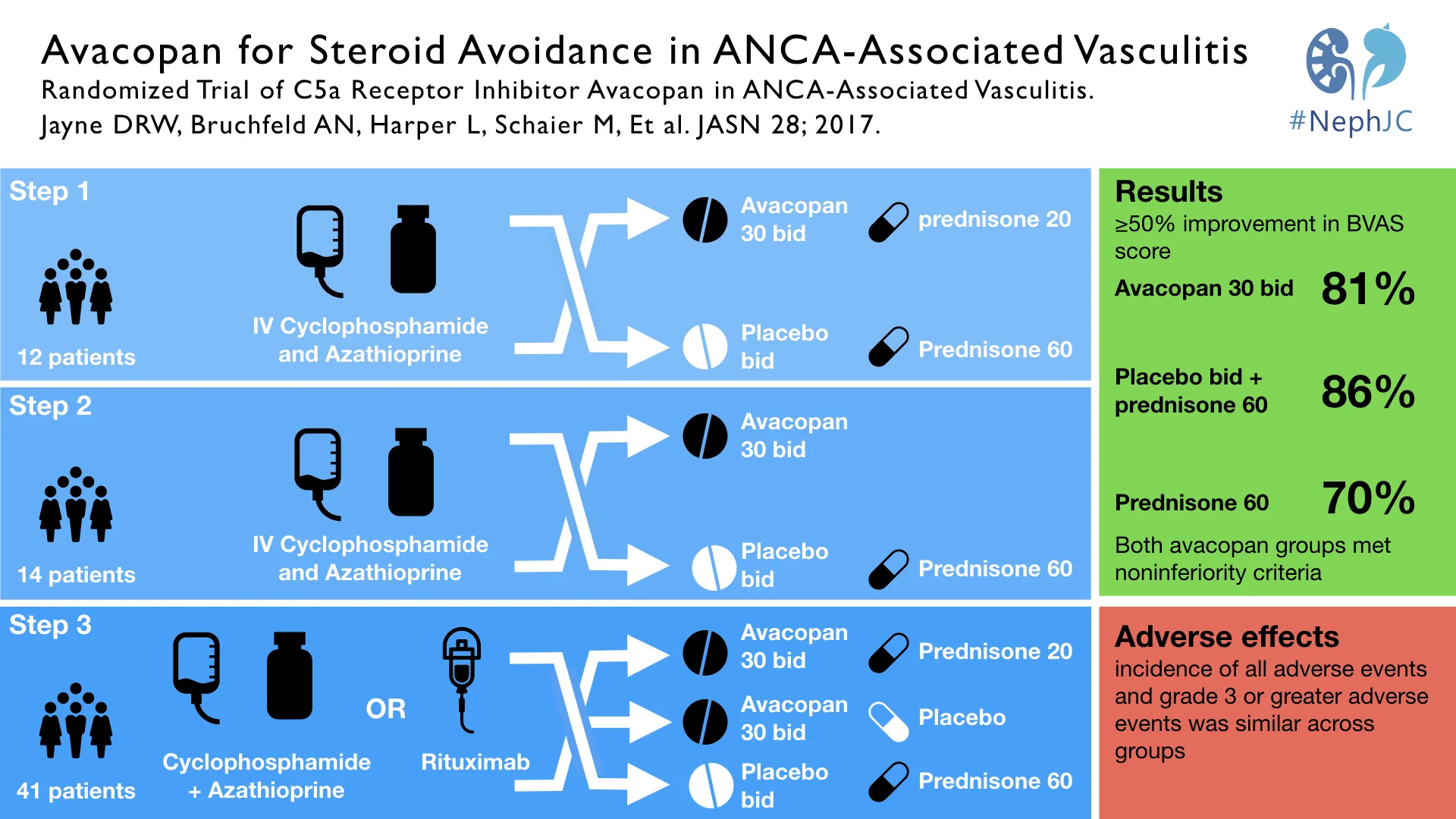
Randomised, phase II, double-blind, placebo-controlled, trial performed in 3 sequential steps:
12 patients initially randomised to placebo + 60mg prednisolone (pred) or Ava + 20mg pred to assess whether Ava could safely allow a GC dose reduction
14 patients randomised to either Ava without pred or placebo + 60mg pred to assess if Ava could replace GC entirely
41 patients were assigned in a 1:1:1 to the groups already listed (placebo + pred; Ava + pred; Ava - pred)
Treatment was for 12 weeks
The efficacy endpoint was only finalised after steps 1 and 2 had been completed
Primary endpoint chosen was a decrease in Birmingham Vasculitis Activity Score of 50% or more
Secondary endpoints chosen included renal response (see table)
Analysis was intention to treat and based on non-inferiority
What did they find?
Efficacy
All three groups were broadly similar at baseline, with most patients representing newly diagnosed disease with an equal split between PR3 and MPO associated diseases
The only key difference between groups was that the placebo + pred group had apparently worse renal disease (higher creatinine) at baseline
“Treatment response” i.e. the primary outcome of 50% reduction in BVAS was seen in a high proportion of all 3 groups. This and other notable secondary outcomes are summarised in the table
Adverse events
Adverse events were similar across all groups, with 17% (4/23) control patients and 25% (11/44) ava patients having serious adverse events
A small number of patients spread across the groups (see table) had worsening vasculitis
Fewer side effects related to GC use were seen in patients on Ava (34% v 65%). This difference was largely driven by fewer patients experiencing psychiatric disorders and new onset diabetes
No deaths were seen
What does it mean?
The top line result for the paper’s authors is that a novel oral C5aR inhibitor can replace high dose GC effectively and safely in patients with AAV. As a result, Chemocentryx have pushed on with a phase 3 trial of avacopan.
This top line result may be true in the long run but how strong is the evidence for it from this paper?
Efficacy
Frank Harrell, one of the doyens of biostatistics, has written at length on the essential invalidity of using observed changes from baseline as a trial endpoint, especially in parallel group studies such as this. In this study both the primary, and a proportion of the secondary, endpoints are presented as percentage change from baseline. The statistically minded amongst you can read more about the problems with this approach here and here, but Harrell’s quote, “The purpose of a parallel-group randomized clinical trial is to compare the parallel groups, not to compare a patient with herself at baseline” is a good summary.
As well as this general objection, there are many basic numeric concerns about using percentage change, such as floor and ceiling effects, and a significant loss of power, which is clearly relevant to a small study such as this. Additionally, patients were treated for only 12 weeks and regression to the mean of assessment scores is another methodological concern with using change scores as a trial outcome over short periods.
As a result, we need to be extremely careful about drawing firm conclusions relating to the efficacy of Ava from this study.
Safety
This short study suggests that Ava was well tolerated and safe when taken for 12 weeks. Especially when side effects potentially attributable to GCs are examined. The curious pattern in lymphopaenia will be interesting to follow in the subsequent phase III study.
What’s the future?
The phase III study is ongoing with an estimated completion date of June 2019. The enrollment target is 300 and the primary endpoint specified is that of disease remission as opposed to a change from baseline, both of which should add greater robustness and interest to the results of the trial.
Summary by Tom Oates