UPDATE 1 (April 9th):
On April 6, 2021 Fibrogen announced that the analysis of the cardiovascular outcomes was adjusted after the data was unblinded. When the data was reanalyzed without this adjustment roxadustat is less impactful. Importantly for this NephJC discussion, the previously impressive MACE and MACE+ advantage for incident dialysis is no longer found. Roxadustat remains non-inferior in all outcomes, though the confidence intervals have widened.
UPDATE 2 (May 17th):
We have news from the Retraction watch blog that both these papers are in the process of retraction. We will update this page once that happens. Keep that in mind while you read anything written below.
#NephJC chat
Tuesday February 23rd 9 pm Eastern
Wednesday February 24th 9pm IST, 9pm GMT
Kidney International Reports 2020 DOI: https://doi.org/10.1016/j.ekir.2020.12.018
Pooled Analysis of Roxadustat for Anemia in Patients with Kidney Failure Incident to Dialysis
and
Kidney International Reports 2020 DOI:https://doi.org/10.1016/j.ekir.2020.11.034
Roxadustat for CKD-related Anemia in Non-dialysis Patients
Daniel W. Coyne, Simon D. Roger, Sug Kyun Shin, Sung Gyun Kim, Andres A. Cadena, Moustafa A. Moustafa, Tak Mao Chan, Anatole Besarab, Willis Chou, Charles Bradley, Meraf Eyassu, Robert Leong, Tyson T. Lee, Khalil G. Saikali, Lynda Szczech, Kin-Hung P. Yu
Introduction
Oxygen makes up 21% of the air we breathe and its availability is essential for life. Otto Warburg won the 1931 Nobel Prize in Physiology or Medicine for explaining how mitochondria enzymatically use oxygen for energy production. Fast-forward 88 years, and oxygen has once again entered the Nobel Prize spotlight. After decades studying the physiological adaptations to hypoxia, Gregg Semenza, Peter Ratcliffe and William G. Kaelin Jr won the 2019 Nobel Prize in Physiology or Medicine for discovery of a protein complex which regulates erythropoietin. Semenza named their newly discovered protein complex the hypoxia-inducible factor (HIF). HIF is a regulatory molecule that enhances renal and hepatic erythropoietin production in response to hypoxic conditions and is now being used to treat anemia. This blog post on the Renal Fellow Network by Arun Kumar covers the physiology:
Anemia is common in kidney disease patients and is associated with increased cardiovascular mortality (Palaka et al, Int Jour Neph 2020). Current treatment strategies include iron supplementation, RBC transfusions and exogenous erythropoiesis stimulating agents (ESAs).
Development and broad utilization of ESAs has led to a dramatic decrease in the need for blood transfusions, but several studies have raised safety concerns due to increased risk for cardiovascular events, especially when aiming for higher hemoglobin (Hb) targets.
USRDS data
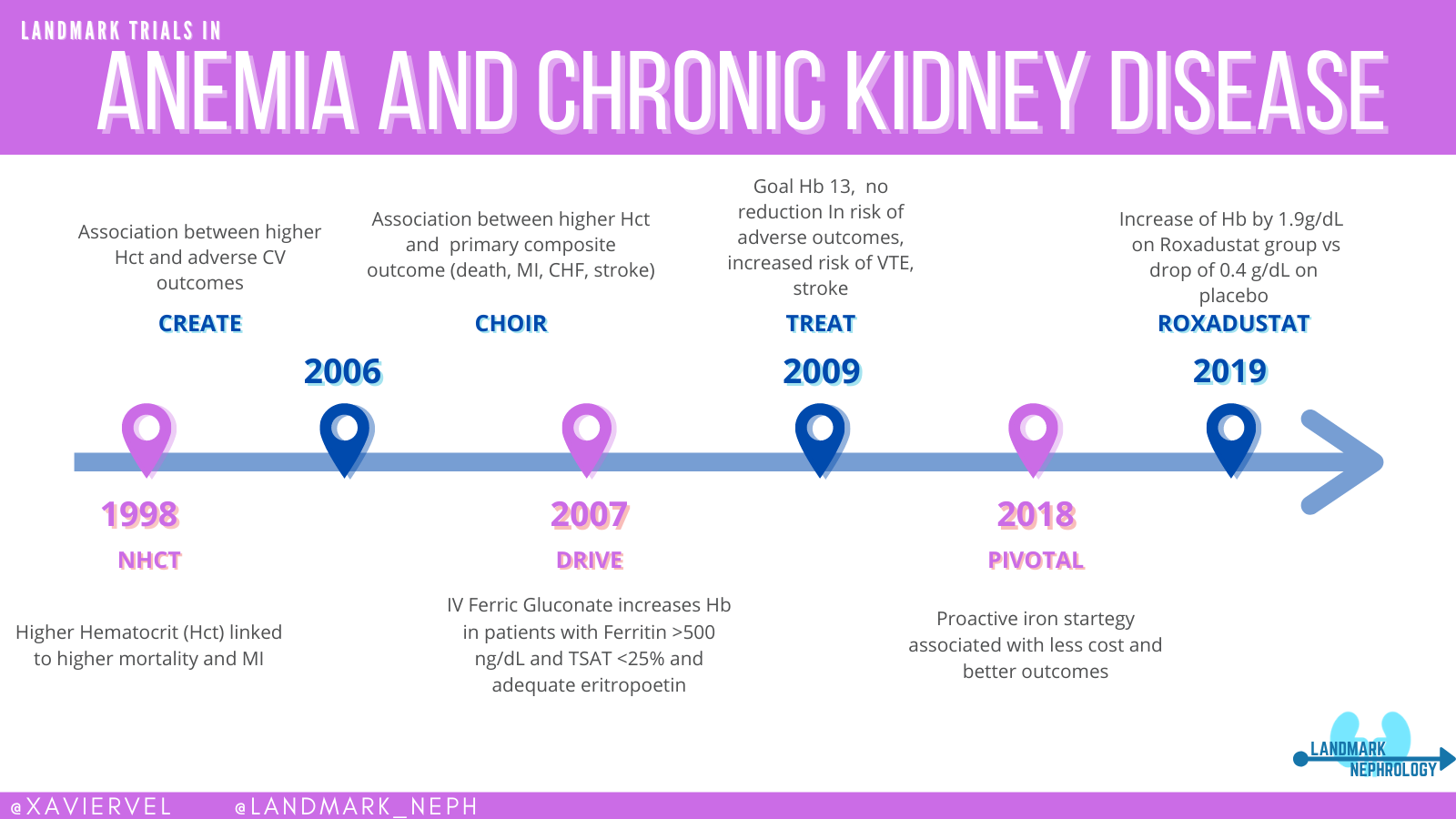
The CHOIR study (Singh et al, NEJM 2006) demonstrated that using Epoetin Alfa in CKD patients to a goal hemoglobin of 13.5 g/dL as compared to 11.3 g/dL lead to an increased risk of severe adverse events without improvement in patients’ quality of life. The CREATE trial (Drueke et al, NEJM 2006) demonstrated a similar increase in serious adverse events without reducing the risk of cardiovascular events in patients treated with ESAs to a normalized hemoglobin target (13.0 to 15.0 g/dL) versus a subnormal target (10.5 to 11.5 g/dL). A similar story of higher cardiovascular morbidity was shown (but buried) much earlier with the Normal Hematocrit trial as well (Besarab et al, NEJM 1998). Read more about this sorry episode in Dan Coyne’s coverage of this as a misstep for Nephmadness in 2019, and the previous NephJC coverage of the quality of life shenanigans. The actual convoluted story includes misleading post hoc analyses, extrapolations, inaccurate guidelines, and an FDA black box warning. The anemia story is a huge misstep in the nephrology lore.
Besarab et al, NEJM 1998
Unlike the previous trials which examined giving an ESA to both groups targeting different hemoglobins, the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT, Pfeffer et al, NEJM 2009) was a placebo-controlled trial, comparing an ESA, darbepoetin, versus placebo (the placebo group could get rescue darbepoetin only when Hb < 9.0). TREAT not only reported no difference in cardiovascular events (questioning a causal relationship between anemia and CV disease, at least at the levels of Hb studied), but in fact reported an increase in stroke in the ESA group (hazard ratio, HR 1.92, 95% CI 1.38 - 2.68). The jury is still out whether it is toxicity due to the ESA - or the higher achieved Hb. This is an important aspect to keep in mind as we examine the newest kids on the block. Check out the Landmark Nephrology post and figure below by Xavier Vela on anemia for a quick overview of the story so far.
Given the nearly 25% first-year mortality for patients initiated on dialysis, strategies that can treat anemia without increasing cardiovascular risk are needed. Roxadustat is one of several prolyl-hydroxylase inhibitors (PHI), along with daprodustat, vadadustat, desidustat, and molidustat, that act by inhibiting hypoxia-inducible factor prolyl hydroxylase, stabilising HIF-α, increasing erythropoietin, and preventing its breakdown. See this table from a nice review (Gupta et al, AJKD, 2017) for some key aspects of the different -dustats.
Phase 3 trials have shown that PHIs improve hemoglobin levels in CKD patients not on dialysis. Roxadustat is approved for CKD-related anemia in China and Japan. But in order to get approval in the US, the FDA demanded cardiovascular safety trials.
One of tonight’s studies pools data from three phase 3 clinical trials of roxadustat in patients on dialysis in order to assess its efficacy and cardiovascular safety compared to epoetin alfa.
The other, ANDES (A Study of FG-4592 for The Treatment of Anemia in Chronic Kidney Disease Patients Not Receiving Dialysis), is a multi-center, global, phase III trial of roxadustat that compares it to placebo for the treatment of CKD-associated anemia in patients not on dialysis.
Study #1: Pooled Analysis of Roxadustat for Anemia in Patients with Kidney Failure Incident to Dialysis
Design
Data was pooled from 3 randomized, multicenter, open-label, epoetin alfa-controlled phase 3 studies which were registered on clinicaltrials.gov (NCT02052310, NCT02273726, NCT02174731).
Study Population
Inclusion criteria
Inclusion criteria were slightly different among the 3 studies pooled, however the important inclusion criteria are:
Patients are newly initiated on hemo- or peritoneal dialysis within 4 months of randomization
Mean Hb between 10 and 12 g/dL
Ferritin over 100 ng/ml and TSAT over 20%
18 years of age or older
Weight under 160 kg
Exclusion criteria
A red blood cell transfusion within the last 4-8 weeks
Intervention
Patients were randomized (1:1) to receive either oral roxadustat or parenteral epoetin alfa. Starting Roxadustat dosing was 70 mg thrice weekly (TIW) for patients weighing ≤70 kg and 100 mg TIW for patients weighing over 70 kg. The maximum roxadustat dose was 3.0 mg/kg per dose or 400 mg per administration (whichever was lower). Epoetin alfa was dosed according to the country-specific product labeling and doses were adjusted to maintain hemoglobin level at 10.0–11.0 g/dL (US cohort) or 10.0–12.0 g/dL (ex-US cohort).
Trial endpoints
Primary US efficacy endpoint: mean change in hemoglobin from baseline averaged over weeks 28–52 regardless of rescue therapy
Primary EU efficacy endpoint: mean change in hemoglobin from baseline averaged over weeks 28–36 without rescue therapy within 6 weeks of and during the 8-week evaluation period
Primary cardiovascular safety endpoint: time to first MACE, a composite measure of myocardial infarction (MI), stroke, and all-cause mortality (ACM)
Secondary cardiovascular safety endpoints:
MACE+: a composite endpoint of MI, stroke, ACM, hospitalization for unstable angina, and hospitalization for congestive heart failure (CHF)
MACE CV mortality: death due to a CV cause, MI, or stroke
MACE+ CV mortality: death due to a CV cause, MI, stroke, ACM, hospitalization for unstable angina, or hospitalization for CHF
Statistical analysis
The trial was powered for non-inferiority (if that is perplexing, check out this NephJC Stats explainer from Manasi Bapat)
The primary efficacy analysis used a multiple imputation analysis of covariance (ANCOVA) model, including terms for treatment group, baseline hemoglobin, and stratification factors
The primary analysis of time to first MACE used a Cox regression model to obtain the hazard ratio of roxadustat versus epoetin alfa
Funding
This study was sponsored by FibroGen, Inc. Employees and subcontractors from Fibrogen had a role in designing the study, data collection, data analysis, data interpretation. Six of the manuscript authors are Fibrogen employees.
Results
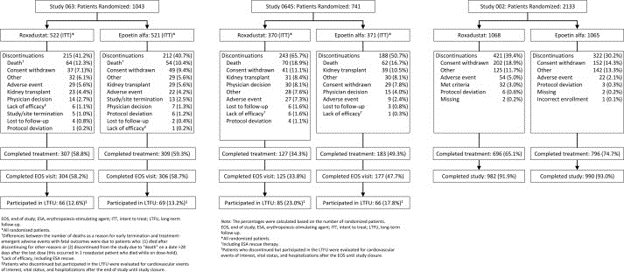
A total of 3917 were randomized in the 3 trials (see CONSORT flow diagram below). Of these, 1530 patients met the criteria of newly initiated on dialysis within 4 months of randomization and were included in the intent-to-treat (ITT) analysis. 760 in the roxadustat group and 770 in the epoetin alfa group.
CONSORT flow diagram from the 3 studies:
Of the 1530 patients included in the final analysis:
61% were men with an average age of 53.8 years
66% were white
79% were iron replete (ferritin ≥100 ng/ml and TSAT ≥20%)
42% had diabetes mellitus
43% had a history of cardiovascular disease
89% were on hemodialysis
Primary Efficacy Endpoints
US efficacy endpoint: The least squares mean analysis was 0.22 (95% CI: 0.05-0.40); P=0.0130 demonstrating that roxadustat was non-inferior and superior to epoetin alfa with a significantly large increases in hemoglobin levels from baseline averaged over weeks 28–52.
EU efficacy endpoint: The least squares mean analysis was 0.28 (95% CI: 0.11-0.45); P=0.0013 demonstrating that roxadustat was non-inferior and superior to epoetin alfa with a significantly large increases in hemoglobin levels from baseline averaged over weeks 28–36.
Secondary Efficacy Endpoints
There was a reduction in mean monthly intravenous iron use over weeks 28-50 with 53.6 mg patient-exposure months in the roxadustat group versus 70.2 mg patient-exposure months in the epoetin alfa group (P<0.0001).
There was no significant difference in patients receiving RBC transfusions or having exacerbation of hypertension.
Primary Cardiovascular Safety Endpoint
Over a mean of 1.4 years of treatment exposure in the roxadustat group and 1.6 years of treatment exposure in the epoetin alfa group, there was a lower risk for MACE in the roxadustat group, hazard ratio of 0.70 (95% CI: 0.51-0.96; P=0.029).
Secondary Cardiovascular Safety Endpoint
The risk for MACE+, MACE+ CV mortality and MACE CV mortality were all significantly lower in the roxadustat group versus the epoetin alfa group. Thus overall, roxadustat was non-inferior (see 95% CI do not touch vertical dotted line below), and for some outcomes, roxadustat was superior (when the 95% CI do not touch the solid vertical line).
Adverse Events
A treatment emergent serious adverse event occured in 42% of patients in the roxadustat group and 42% patients in the epoetin alfa group.
We will discuss the implications of this together with the next study.
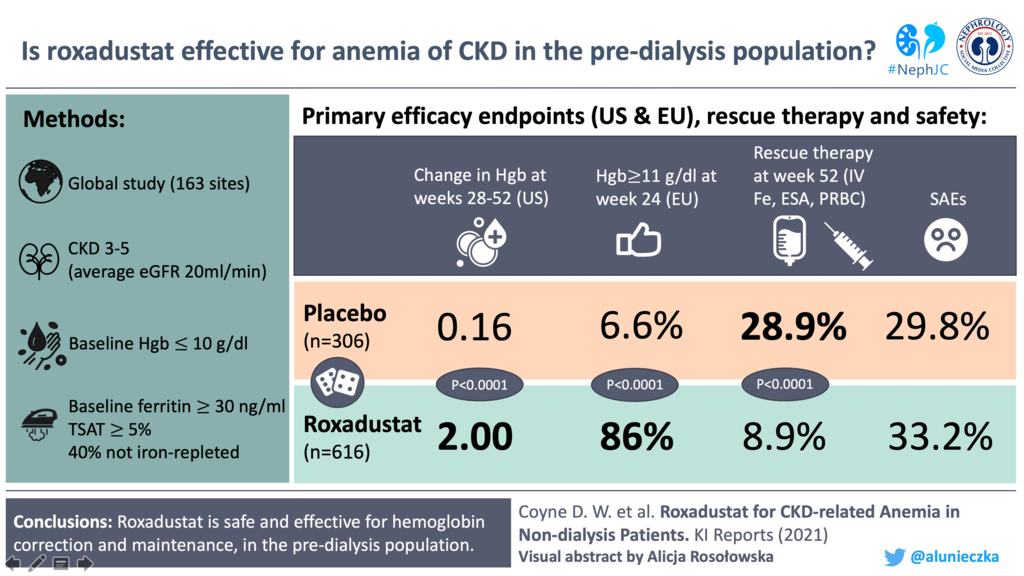
Study #2: Roxadustat for CKD-related Anemia in Non-dialysis Patients
Design
ANDES is a global, multi-center, phase III, randomised, double-blinded, placebo-controlled trial conducted at 162 sites in the United States, South America, Australia, New Zealand, and Asia. (ClinicalTrials.gov number, NCT01750190)
Study Population
Inclusion criteria
Aged ≥18 years
KDOQI Stage 3, 4, or 5, and not receiving dialysis
Mean of the 3 most recent hemoglobin values below 10 g/dL
Body weight 45–160 kg
Ferritin over 30 ng/mL (≥66 pmol/L) and TSAT over 5%
Serum folate level and vitamin B12 above the lower limit of normal
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) less than 3× the upper limit of normal, and total bilirubin less than 1.5× the upper limit of normal
Exclusion criteria
AEecent dose of ESA, IV iron, or RBC transfusion
Active infection
History of chronic liver disease
CHF, MI, stroke, hypertension or other cardiovascular events within 12 weeks of randomization
Intervention
Patients were randomly assigned (2:1) to receive oral roxadustat or matching placebo. Patients weighing 45 to 70 kg received 70 mg roxadustat or placebo, and those weighing over 70 kg received 100 mg thrice weekly. Randomization was stratified by:
Screening hemoglobin (≤ 8.0 vs. > 8.0 g/dl)
History of cardiovascular, cerebrovascular, or thromboembolic diseases
eGFR (<30 vs. ≥30 ml/min per 1.73 m2)
Region (US vs. countries outside US)
Sample size
A minimum of 450 and up to 1200 subjects were planned to be randomized in a 2:1 ratio to either roxadustat (≥300 and ≤800 patients) or placebo (≥150 and ≤400 patients) in a double-blind manner. The study was sufficiently powered for both regionally-based primary efficacy endpoints.
Statistical Analysis
The primary analysis are expressed below in all of their statistical gobbledygook, but in simple English, they were looking at the change in hemoglobin.
For the US FDA primary endpoint, a missing-at-random-based multiple imputation analysis of covariance model tested the null hypothesis that hemoglobin changes from baseline averaged over weeks 28 to 52 regardless of rescue therapy were comparable in roxadustat and placebo patients in the intent-to-treat (ITT) population (all randomized patients).
For the EU European Medicines Agency primary endpoint, a Cochran-Mantel-Haenszel model tested the null hypothesis that the percentage of patients achieving a hemoglobin response without rescue therapy would be comparable in roxadustat and placebo patients in the full analysis set (FAS; all randomized patients who received ≥1 dose of study drug with baseline and ≥1 post-dose hemoglobin).
Outcomes
Primary Outcomes
US primary endpoint: the mean (SD) haemoglobin changes from baseline to weeks 28 to 52 regardless of rescue therapy in the roxadustat versus placebo group using the least-squares mean (LSM) between-group treatment difference.
EU primary endpoint: the proportion of patients achieving a response without rescue therapy at 2 consecutive visits during the first 24 weeks in the roxadustat versus placebo group
Secondary Outcomes
Mean (SD) change in hemoglobin over weeks 28 to 36 without rescue therapy within 6 weeks of and during the 8-week treatment period in roxadustat and placebo groups
Mean (SD) changes from baseline in hemoglobin over weeks 28 to 52 in roxadustat and placebo patients with baseline high-sensitivity C-reactive protein greater than the upper limit of normal.
The proportion of patients with hemoglobin ≥10.0g/dl over weeks 28 to 36 without rescue therapy within 6 weeks of and during the 8-week treatment period in the roxadustat versus placebo group
The mean (SD) change from baseline in LDL cholesterol over weeks 12 to 28 in the roxadustat and the placebo group
Additional, exploratory efficacy endpoints included the following: mean change from baseline in hemoglobin during weeks 28–36 by baseline iron status regardless of rescue therapy; mean changes from baseline in serum hepcidin, iron, and other iron-related parameters, including ferritin, TSAT, and total iron-binding capacity (TIBC), total cholesterol, and low-density lipoprotein (LDL)/high-density lipoprotein ratio at each scheduled visit to week 52.
Funding
Fibrogen funded the study. Astellas Pharma and Astra-Zeneca contribute to the clinical development of roxadustat. FibroGen employees and subcontractors had a role in various components of the study. Seven of the manuscript authors are FibroGen employees. All authors had full access to all of the study data and had responsibility for the decision to submit the manuscript for publication.
Results
922 patients passed screening and were randomised into Roxadustat (616) ITT group and Placebo (306) ITT group. In the roxadustat group, 43% (267/616) of patients discontinued treatment mainly due to adverse events or consent withdrawal; whereas 68% (208/306) of placebo-treated patients discontinued treatment owing to lack of efficacy.
Baseline characteristics between the two groups as seen in the table 1 below. The mean age was 65 years, with ~ 60% women, a mean eGFR of about 20, and a Hb of about 9 g/dL.
Primary Efficacy Endpoints
US primary endpoint: For the weeks 28 to 52, the least-squares mean (LSM) between-group treatment difference (roxadustat-placebo) was 1.85 g/dl (95% CI 1.74–1.97; P < 0.0001) regardless of rescue therapy. The results were consistent across various subgroups.
EU primary endpoint: The proportion of patients achieving a response without rescue therapy at 2 consecutive visits during the first 24 weeks between the roxadustat versus placebo groups was (86.0% [95% CI 83.0%–88.7%] vs. 6.6% [95% CI 4.1%– 9.9%]), with an odds ratio of 77 (95% CI 44.7–134.5; P < 0.0001). Among subgroups also, the results were consistent.
Secondary Efficacy Endpoints
Mean baseline hepcidin levels were comparable in the roxadustat and placebo groups (111 and 106 μg/l). Roxadustat-treated patients experienced dramatic lowering in serum hepcidin levels by week 4 which was partially sustained through week 44. At week 44, the mean changes from baseline was −22 (80.90) μg/l in the roxadustat group and and 4 μg/l in the placebo group with an LSM difference of −26 μg/l (95% CI −39 to −13).
Mean (SD) changes from baseline in hemoglobin over weeks 28 to 52 in roxadustat and placebo patients with baseline high-sensitivity C-reactive protein greater than the upper limit of normal were 2.0 (0.9) and 0.2 (0.9) g/dl, with a LSM difference of 1.9 g/dl (95% CI 1.7–2.1; P < 0.0001)
The mean (SD) change from baseline in LDL cholesterol over weeks 12 to 28 was 18 (30) in the roxadustat group and 0.2 (29) mg/dl in the placebo group, corresponding to an LSM difference of 17 mg/dl (95% CI 21 to 14; P < 0.0001).
Tolerability
Through 52 weeks of treatment, the overall incidence of treatment-emergent adverse events (88% and 86%) and serious adverse events (33% and 30%) in roxadustat and placebo treated patients were comparable. The most common treatment-emergent adverse events were hyperkalemia, constipation, viral upper respiratory tract infection, upper respiratory tract infection, and hypertension.
Discussion
Study #1
The United States Renal Data System’s Annual Data Report in 2018 reported a 78% first-year survival rate for patient’s initiating dialysis. Given the high first year mortality of nearly 1 in 4, minimizing this mortality is a major goal in dialysis. The authors of the first study used roxadustat to treat anemia without increasing the risk of cardiovascular adverse effects and actually reducing the risk for MACE compared to epoetin alfa.
Strengths:
Study size: Study included a large patient population of 1530 patients
International: 48% of patients from Europe, 25% of patients from the USA and 27% from other countries
Limitations of the study:
Short study period: Anemia is a usually long-term issue in CKD/ESKD patients and this trial only provides short-term safety data up to 36 weeks
New dialysis patients: Study demonstrated efficacy of HIF inhibition in incident dialysis patients but unclear if benefit still applicable to patients on prevalent dialysis
Non-inferiority creep: Roxadustat was non-inferior to epoietin alfa, but as we have seen above (eg. TREAT), the ESA may itself be inferior to placebo in terms of CV safety esp stroke. So is this a valid comparison?
Study #2
In the second study for non–dialysis-dependent CKD patients with anemia, the trial demonstrated roxadustat to be superior to placebo in improving the haemoglobin levels and decreasing the need for RBC transfusions. Roxadustat also decreased LDL levels and improved the iron profile in comparison to the placebo-controlled group. The safety profile was comparable to placebo and consistent with patients with CKD-related anemia.
Since iron is essential for RBC production even in patients receiving ESAs, a patient’s iron status and other factors affecting the availability of iron need to be considered when assessing anemia treatment. A great example of this is the PIVOTAL trial which demonstrated that proactively administering higher doses of intravenous iron was associated with a reduction in the incidence of the primary cardiovascular endpoint along with lower doses of erythropoiesis-stimulating agents, however this study was performed in a hemodialysis population. KDIGO recommends iron supplementation for CKD patients with a TSAT ≤30% and ferritin is ≤500 ng/ml, however in this trial, the “iron replete” designation only required TSAT to be ≥20% and ferritin ≥100 ng/ml. Furthermore, less than 60% of patients met even that lower threshold for being considered “iron replete”. As such, it may be beneficial for the authors to perform a subgroup analysis assessing the efficacy of roxadustat on the portion of patients that had a TSAT ≥30% and a ferritin ≥500 ng/ml.
One interesting iron-related factor that this trial did a good job capturing was the effect roxadustat had on hepcidin. Hepcidin is a protein that regulates both intestinal iron absorption and iron mobilization from hepatocytes and macrophages (Nemeth and Ganz, Annu Rev Nutr 2006). Lower hepcidin levels increase iron accessibility needed for RBC production. Hepcidin levels decreased by week 4 and remained suppressed through week 44. This represents an additional mechanism by which PHI improves anemia.
Strengths:
Use of placebo against roxadustat reflects current standard of care as usage of ESAs have decreased since showed an association between these drugs and cardiovascular risk.
The trial had a lower mean baseline eGFR in comparison to previous trials (CHOIR, CREATE) that allowed studying a population not covered previously.
Limitations:
The iron status of patients in the trial was not optimized and the authors’ definition of “iron replete” is questionable as it required TSAT to be ≥20%. Even at that lower threshold, only about 60% of the patients were considered iron replete.
Apart from these published data, we do have some information from the ASN Kidney Week presentation, as can be seen below, on the CV safety in other settings (slide from Robert Provenzano’s presentation at a recent webinar). Though the data is still not published, it provides a better overview of the CV safety across the CKD spectrum.
If these drugs get approved, should we start using them? An oral option for the non-dialysis CKD population and for home dialysis patients would be a major improvement for these patients. They also look like interesting options in the setting of inflammation-mediated iron sequestration. Since erythropoiesis is limited by the availability of iron to produce new RBCs, the combination of increasing iron availability through decreased hepcidin levels and increased endogenous erythropoietin may provide the one-two punch needed for patients with difficult-to-treat anemia.
Given that modulation of the oxygen-sensing pathway is likely to influence the expression of hundreds of genes, there are likely many developmental, physiological and pathological processes that PHIs affect which we have yet to identify. As such, the safe cardiovascular risk profiles from these initial studies are encouraging.
Conclusion
The discovery of the oxygen sensing pathway’s role in anemia has opened the door for alternative treatment strategies for treating CKD-related anemia. The current studies provide evidence that hypoxia-inducible factor stabilization with PHI-inhibitors can treat CKD-related anemia.
Summary prepared by
Priyam Gupta,
Junior Resident at Shri Lal Bahadur Shastri Govt. Medical College & Hospital, India
Deep Phachu,
Nephrology Fellow at the University of Connecticut
NSMC interns, class of 2021