#NephJC Chat
Sep 2 2025 9 pm EST
Lancet. 2025 Aug 16;406(10504):695-704. doi: 10.1016/S0140-6736(25)01198-5.
Spironolactone versus placebo in patients undergoing maintenance dialysis (ACHIEVE): an international, parallel-group, randomised controlled trial
Michael Walsh, David Collister, Martin Gallagher, Patrick B Mark, Janak R de Zoysa, Jessica Tyrwhitt, Karthik Tennankore, Gilmar Reis, Denis Xavier, Wen J Liu, Li Zuo, Amanda Y Wang, Camilo Félix, Laura Sola, Mustafa Arici, Russell Villanueva, Vivekanand Jha, Dalton Précoma, Christian G Rabbat, Sheik Sulthan Alavudeen, Atiya R Faruqui, Mavel López-Flecher, Lonnie Pyne, Ron Wald, Fei Yuan, Kumar Balasubramanian, Shun Fu Lee, Alena Kuptsova, Courtney Christou, P J Devereaux; ACHIEVE Investigators
PMID: 40818850
Protocol Paper: Walsh et al Can JKHD 2025
Introduction
Cardiovascular disease, mostly from arrhythmias/cardiac arrest, is the leading cause of morbidity and mortality in dialysis patients, a fact that has remained unchanged for decades, highlighting the need for effective preventive strategies (see figure below from the 2024 USRDS report). Dialysis patients have a 14-fold increased risk of mortality compared to the general population, and nearly 40% of dialysis patients die within 3 years of dialysis initiation. (Wouter J, et al. Circulation 2019) Indeed, mortality observed in the dialysis population is much higher than some types of solid cancers, which receive much greater recognition, financial support, and therapeutic innovation. (Kyla LN,et al. AJKD 2018)
Percentages of cause-specific mortality with and without inclusion of missing and unknown causes of death in patients with ESRD receiving hemodialysis who died in 2022, data source: 2024 USRDS report.
Aldosterone, through genomic and non-genomic effects, has a significant role in precipitating left ventricular hypertrophy (LVH), increasing the left ventricular mass index (LVMI), promoting coronary artery disease (CAD), and cardiac fibrosis. Previous studies have highlighted the association of high aldosterone (>200 pg/ml) with an elevated risk of mortality in dialysis patients. (Christiane D, et al. EHJ 2013) Relative hyperaldosteronism is frequently observed as a consequence of volume expansion and inadequate suppression of the renin–angiotensin–aldosterone system (RAAS) in CKD and ESRD patients. These persistently elevated aldosterone levels are thought to be an important contributor to the high burden of cardiovascular events via various ‘pleiotropic’ effects. It is unclear what effect the RAAS system actually has in anuric dialysis patients with non-functioning nephrons.
Mineralocorticoid receptor antagonists reduce myocardial fibrosis, LVH, and left ventricular stiffness. They improve myocardial diastolic and systolic function, thus reducing sudden cardiac death and heart failure deaths in specific non-dialysis populations, mostly in those with heart failure.
In the non-dialysis CKD population, MRAs, particularly finerenone, have been reported with favorable kidney and CV outcomes (FIGARO and FIDELIO), and sadly good spironolactone trials have not been done. Specifically with ESRD however, there have been many small trials with fantastically fabulous results. The majority of these trials in ESRD patients had significant limitations due to unclear allocation, high loss to follow-up, and small event numbers, as shown in this systematic review (Quach K, et al. AJKD 2016). Pooling all trials with no regard to study quality and risk of bias gives us a 60% reduction in CV outcomes and all-cause mortality (when CV disease - mostly arrhythmias only make up a paltry ~ 30% of deaths). Surely spironolactone cannot reduce infection-related deaths and rates of withdrawal from dialysis? Nevertheless, in the face of these impressive prior trials, a large robust trial was necessary. The ACHIEVE trial was this attempt to answer the question of whether spironolactone has a positive impact on cardiovascular outcomes in incident patients on dialysis.
The Study
Methods
Study design
The Aldosterone Blockade for Health Improvement EValuation in End-stage Renal Disease (ACHIEVE) trial was a multi-center, international, parallel-arm RCT in which the effects of spironolactone versus placebo were compared in patients undergoing maintenance dialysis.
Study population
Patients were enrolled from 143 dialysis programs from 12 countries (Australia, Brazil, Canada, China, Ecuador, India, Malaysia, New Zealand, the Philippines, Turkey, the UK, and Uruguay).
Run-in period
After screening and consent, participants entered an open-label active run-in period in which they received spironolactone by mouth for 7 to 14.5 weeks. Spironolactone 25 mg was chosen because it is similar to doses used in previous trials of spironolactone in patients with heart failure without kidney disease and because it appeared effective in other, smaller trials involving patients with kidney failure. Since spironolactone can cause hyperkalemia - and if participants in the intervention arm get their active drug stopped within weeks from this reason such that very few will remain on it for the trial duration (as happened in BARACK-D), the run-in period was very important. See the NephTrials summary of this for more details.
Interventions
Adherence in the run-in was defined as having taken at least 80% of the study medication by self-assessment, with a serum potassium ≤6 mmol/L, and willingness to continue. Those who were adherent were randomly allocated to continue spironolactone 25 mg daily or a matching placebo (1:1). Spironolactone was initiated at 25 mg three times weekly due to safety concerns, with the option to increase to daily dosing. The use of potassium binders, exchange resins, or adjustments in dialysate potassium was left to the discretion of local physicians. Random allocation was accomplished using a concealed, random number list prepared by an independent statistician with randomly permuted block sizes, stratified by center, using an interactive web-based randomization system.
Outcomes
Primary Outcome
The primary outcome was time to first composite of cardiovascular death or hospitalization for heart failure.
Secondary Outcomes
Time to cause specific death (cardiac, vascular, non-cardiovascular)
Time to first hospitalization for heart failure
All-cause death
Time to first all-cause hospitalization
Severe hyperkalemia
Tertiary and exploratory outcomes were:
stroke (fatal or non-fatal)
myocardial infarction (fatal or non-fatal)
new onset atrial fibrillation
reason for discontinuation of study medication
health-related quality of life
dialysis recovery time
hospitalization by system organ class.
Adjudication
Blinded adjudication with strict predefined criteria: HF hospitalization required symptoms plus fluid removal; CV death assumed unless a clear non-CV cause was documented; out-of-the-hospital deaths classified using structured interviews with family and caregivers.
Statistical analysis
Analysis included all participants in the group to which they were randomly assigned, irrespective of treatment received, and follow-up time was censored at the time of kidney transplantation, death, or last follow-up. Time to first event analyses, excluding all-cause mortality, were conducted using cause-specific Cox regression models accounting for the competing risk of non-cardiovascular deaths. The study aimed for 700 primary events, with initially planned 2750 participants. Since this was an event driven trial, the sample size was revised in April 2023 for higher adherence, allowing for 650 events to be sufficient. Recruitment proved slower due to the impact of the COVID-19 pandemic.
Funding
The Canadian Institutes of Health Research, The Medical Research Future Fund, The Health Research Council, The British Heart Foundation,
Population Health Research Institute/Hamilton Health Sciences Research Institute, St Joseph’s Healthcare Hamilton Division of Nephrology, Accelerating Clinical Trials Consortium, Can SOLVE CKD Network, and the Dalhousie Department of Medicine. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The study was conducted between September 2017 and October 2020. A total of 3,689 patients were screened, of whom an impressive 3565 were enrolled in the run-in phase, and 2,538 participants were randomized to receive either spironolactone (n=1260) or placebo (n=1278). Note the detailed exclusions after run-in period, including ~ 500 for non-adherence and ~ 370 for hyperkalemia, and sadly 59 dying during the run-in period itself, underscoring the high mortality in dialysis.
Figure 1. Trial profile, from Walsh M, et al. Lancet, 2025
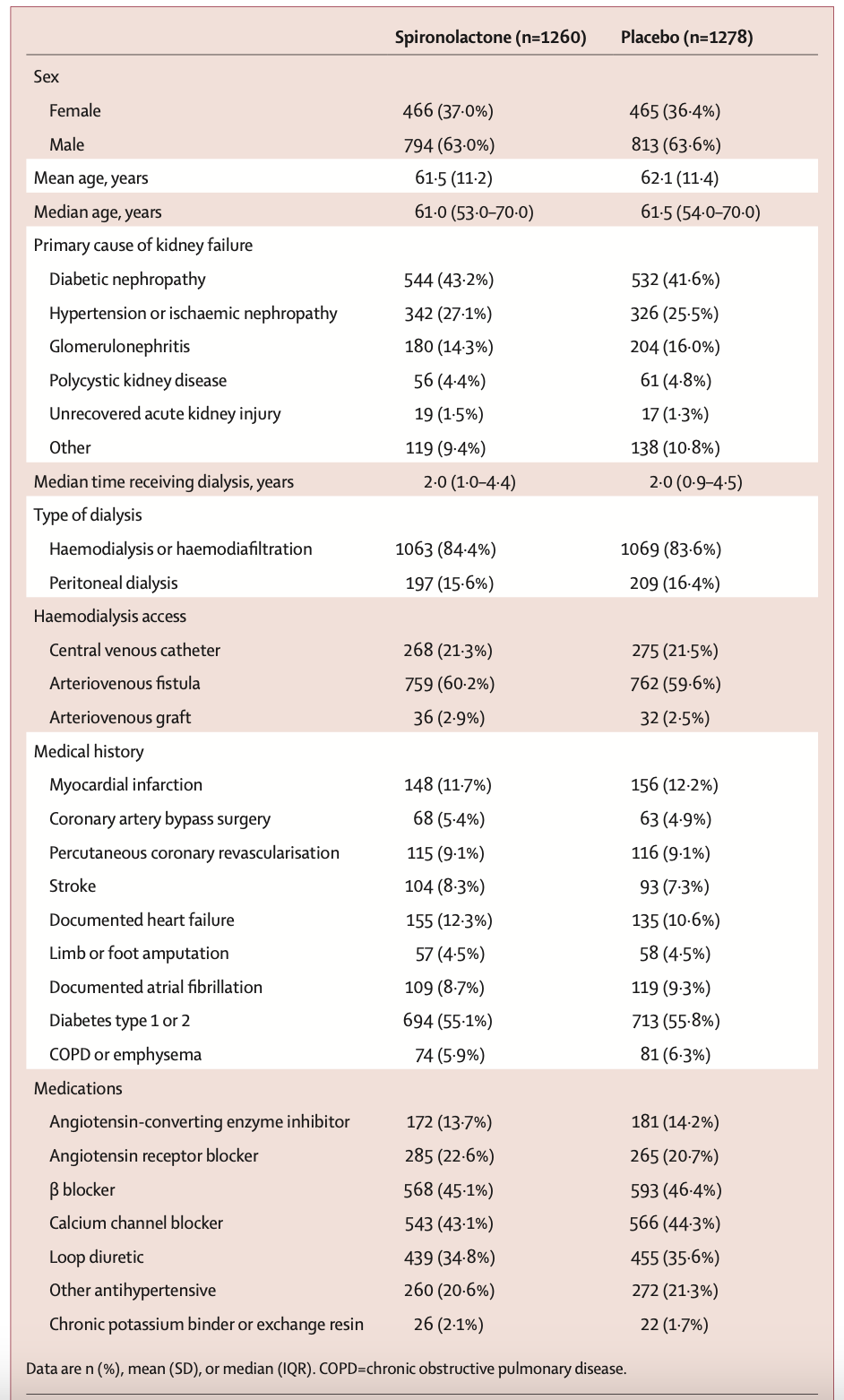
Approximately 63% of participants were male, and the mean age was 62 years. Overall, 43% of patients had diabetic kidney disease. Most patients (84%) were receiving hemodialysis or hemodiafiltration, and about 60% had arteriovenous fistulas. In terms of concomitant medications, about one-third were treated with RAS inhibitors, another third with diuretics, and 2% with potassium binders. Median vintage of dialysis was ~ 2 years.
Table 1. Baseline characteristics, from Walsh M, et al. Lancet, 2025
Primary outcome
After reviewing interim data, which included 508 reported primary outcome events (78% of the total expected), the external safety and efficacy monitoring committee recommended stopping the trial for futility, based on a conditional power analysis.
Figure 2. Cumulative incidence of primary outcome, from Walsh M, et al. Lancet, 2025
During a median follow-up of 1.8 years, the composite primary outcome—cardiovascular death or hospitalization for heart failure occurred in 258 participants in the spironolactone group (10 events per 100 patient-years) and in 276 participants in the placebo group (11 events per 100 patient-years), corresponding to a hazard ratio of 0.92 (95% CI 0.78–1.09). These results were robust in different sensitivity analyses (table S2) accounting for transplanted participants, centre effect, and a win ratio analyses.
Subgroups
The subgroup results were overall consistent, except for a bizarre and most likely spurious difference by sex, in the opposite direction of what one would presuppose (since men are more likely to discontinue spironolactone due to the anti-androigenic adverse effects).
Figure 3. Primary composite outcome events (cardiovascular death or hospitalisation for heart failure) by subgroup, from Walsh et al, Lancet 2025
Secondary Outcomes
Secondary outcomes showed no statistical differences between the spironolactone and placebo groups. The risks of death—whether from cardiac, vascular, non-cardiovascular causes, or from any cause—were similar. Likewise, there were no substantial differences in the time to first hospitalization for heart failure, the total number of hospitalizations for heart failure, the time to first hospitalization for any cause, or the total number of hospitalizations. Only one of the secondary outcomes was significant: ~ 50% higher severe hyperkalemia rates with spironolactone, despite excluding ~ 370 participants in the run-in period from hyperkalemia.
Table 3. Primary and secondary outcomes, from Walsh M, et al. Lancet, 2025
Adverse Events
Treatment was discontinued in 294 patients in the treatment arm and 241 patients in the placebo arm. Patient preference and hospitalizations were the most common reasons for stopping treatment. With regard to hyperkalemia as a cause for treatment discontinuation, it was more frequent in the spironolactone group (23 participants) compared with the placebo group (11 participants). In addition, the number of severe hyperkalemia episodes was higher in the spironolactone group (123 events) than in the placebo group (80 events).
Analysis of hospitalization causes showed that pneumonia or other lung infections were the most frequent reason for admission, and occurred with similar frequency in the spironolactone group (165 events) and placebo group (177 events). Moreover, since the study was conducted during the COVID-19 period, a slightly higher number of infections was observed in the spironolactone group, though without statistical significance. Hospitalizations due to other non-cardiovascular causes, the second most common reason for admission, were similar in the spironolactone group (208 events) compared with the placebo group (229 events).
Table 2. Adverse effects, from Walsh M, et al. Lancet, 2025
Discussion
Mineralocorticoid receptor antagonists have shown definitive benefits in patients with significant heart failure (NYHA 3/4) without ESRD, achieving decreased hospitalizations and improved cardiovascular outcomes. There are theoretical reasons spironolactone might retain its benefits in patients on dialysis. These theoretical reasons do not matter anymore since in this well-done, large trial, there was absolutely no benefit of spironolactone in ESRD. Coupled with the risk of serious hyperkalemia (despite the active run-in period excluding many who developed hyperkalemia), spironolactone should no longer be used for CV protection in ESRD. It is known by most nephrologists that the physiology of patients with ESRD is unique, even from that of CKD patients. Patients with ESRD have a very high cardiovascular event rate, but this is mostly sudden death and arrhythmia, and it is difficult to fathom how spironolactone would be so useful in this setting, as the previous small and biased trials seemed to suggest. Even if one believes the voodoo of inflammation and cardiovascular remodeling, with LVH and cardiac fibrosis, this would have been going on for years if not decades prior to ESRD, so spironolactone at this stage may be too late. Treatment of the hyperaldosterone state after the initiation of dialysis, where critical left ventricular mass and widespread cardiac fibrosis are already present, seems unlikely to improve with short-term MRA use (the house has burned to the ground long before we threw any water on it). Needless to say, we make these comments with our retroscopic hindsight, and in the presence of the impressive ~60% relative risk reduction in the previous small trials, a large trial like this was justified and necessary to guide clinical practice.
Many other interventions have similarly failed to show as impressive results in ESRD as in the non-ESRD population (e.g. statins, RASi). Does spironolactone even have any effects in the physiology of oliganuric end-stage kidneys? Clearly, the occurrence of hyperkalemia demonstrates some important effects (though this might be mediated by gut aldosterone receptors, see Nakamura T, et al, JAHA 2018). Unfortunately, beyond the absence of significant hypotension, we don’t have any data in this trial of other mechanisms to demonstrate important spironolactone effects. The other main take-home point is the importance of large multicentre trials with a large number of outcome events, which is the only way to have confidence in results.
A notable feature of the ACHIEVE study was the run-in period, which helped identify hyperkalemia in about 10% in the ~ 7 week run-in period. The run-in period also excluded participants with non-adherence, thus enriching the trial population with participants who were adherent and at lower risk of hyperkalemia. Any benefit of spironolactone was much more likely to be seen in this scenario, and spironolactone still flopped.
The subgroup analyses showing a potential effect modification by sex will cause much heartburn and teeth gnashing. Not only is this against what one would expect, but this analysis was also not adjusted for multiple testing. Conversely, a small study in PD has reported a larger effect on LVH in men (Ito et al, JASN 2014) so this finding, though not worthy of practice change, is still intriguing for further research.
Given the pragmatic nature, we do not have BP data, and it may be reasonable to (carefully, monitoring for hyperkalemia) use it for uncontrolled hypertension after exhausting the usual means (beta-blockers, better volume control). Additionally, spironolactone is also sometimes used to prevent hypokalemia in PD, which might be a reasonable 2D indication to continue (Langote et al, PLoS One 2017).
Finally, the concurrent results of the ALCHEMIST trial also showed that spironolactone did not reduce major cardiovascular events (MACE) in older, high-risk hemodialysis patients, further suggesting that the use of spironolactone is not supported for dialysis patients to decrease cardiovascular mortality or heart failure hospitalizations. (Rossengal P, et al. The Lancet 2025)
Limitations
ACHIEVE was a pragmatic trial including almost all patients on dialysis who could tolerate spironolactone, which is both a strength increasing generalizability, but also a limitation in case spironolactone could work in some small ‘higher risk’ subpopulation. This is very unlikely given the large number of events seen in this trial. The run-in period could have been considered a limitation exaggerating spironolactone's effectiveness in case it helped - but it did not, making this a non-issue. A final important limitation is that spironolactone 25 mg daily was the only dosing strategy studied. Different doses, different mineralocorticoid receptor antagonists, or other mechanisms of aldosterone antagonism could have had different effects, though this is extremely speculative. As we have discussed before, most dialysis patients die of sudden death, infections, and withdrawal, and it is implausible to expect a huge effect of spironolactone.
Conclusion
In patients receiving maintenance dialysis, spironolactone did not reduce the composite outcome of cardiovascular mortality and hospitalization due to heart failure, compared with placebo. Time to stop using it for CV benefits in dialysis. Achieve Immortality? Not in dialysis.
Summary prepared by
Reviewed by: