#NephJC Chat
Tuesday, November 18th 2025, 9 pm Eastern on Bluesky
N Engl J Med. 2025 Nov 6. doi: 10.1056/NEJMoa2510198. Online ahead of print.
A Phase 3 Trial of Atacicept in Patients with IgA Nephropathy
Richard Lafayette, Sean J Barbour, Robert M Brenner, Kirk N Campbell, Tom Doan, Necmi Eren, Jürgen Floege, Vivekanand Jha, Beom Seok Kim, Adrian Liew, Bart Maes, Atanu Pal, Roberto Pecoits-Filho, Richard K S Phoon, Dana V Rizk, Hitoshi Suzuki, Vladimir Tesař, Hernán Trimarchi, Xuelian Wei, Hong Zhang, Jonathan Barratt; ORIGIN Phase 3 Trial Investigators
PMID: 41196369
Introduction
IgA nephropathy (IgAN) has seen a spectacular and monumental rise in the elucidation of its pathophysiology, and not surprisingly this is revolutionizing therapeutics as well. We just reviewed the KDIGO 2025 IgAN guidelines before Kidney Week (KDIGO IgA page|NephJC Summary), which, some have noted, already requires updating. Firstly, KDIGO’s framework still treats “progression attenuations” as the ceiling, even as combination therapy is shifting CKD care toward the possibility of remission. The shift is more than theoretical: flozins, sparsentan, target-release budesonide, and combination regimens are showing that kidney trajectories can bend in ways the guidelines did not account for. In IgAN the mismatch is sharper. The guideline was written for an ORIGIN story focused on supportive care; the field now operates with different ambitions and different tools.
Figure 1: Paradigm for CKD remission, from Tangri N, et al. Kidney Int. 2025
The four-hit model gives the mechanistic logic for that shift. Overproduction of Gd-IgA1, development of antiglycan IgG or IgA, immune complex assembly, and the mesangial deposition describe a sequence that is targetable and multiple points. IgA nephropathy is a B-cell mediated immune complex glomerulonephritis. A proliferation-inducing ligand (APRIL) and B-cell activating factor (BAFF) sit upstream of these hits by sustaining B-cell maturation, class switching, and plasma cell survival. Elevated APRIL and BAFF levels correlate with disease severity, Gd-IgA1 levels, and immune-complex burden. Blocking this axis, with agents that neutralize APRIL alone or both cytokines, suppresses Gd-IgA1 synthesis and shrinks the pathogenic pool that drives IgA depositions and downstream inflammation. The immunologic rationale is consistent across the existing empirical evidence. (Zajn, et al. Kidney Int Rep. 2024| Barratt J, et al. Kidney Int Rep, 2022| Tumlin J, et al. WCN24-762, Kidney Int Rep. 2024)
Image 1: BAFF and APRIL in B-cell autoimmunity. Cheung CK, et al. Front Nephrol, 2024.
Atacicept (pronounced atak-ǝh-sept) is a TACI–Fc fusion protein with very high affinity for BAFF and APRIL. TACI (transmembrane activator and calcium-modulator and cytophilin ligand interactor) is a member of the TNF family of receptors and targeting it (by all the -tacicepts in development) allows blocking the activity of both BAFF and APRIL. Blocking these signals suppresses B-cell activity and reduces levels of galactose-deficient IgA1 and circulating immune complexes. This directly targets the specific mechanisms underlying the initiation of IgAN, versus the systemic and non-specific treatments previously employed for the downstream effects of Gd-IgA mediated inflammation.
Figure 1. Role of APRIL in the pathogenesis of IgAN- the tonsil- and gut-kidney axis, from Chacko B et al, Kidney News, 2024
The ORIGIN 2b trial showed that atacicept not only clearly reduced proteinuria in IgAN, but additionally lowered Gd-IgA1, improved hematuria, and helped stabilize eGFR decline. (Lafayette R, et al. Kidney Int. 2024) In the extension study (up to 96 weeks), these benefits continued with almost no further eGFR decline. This suggests atacicept may be a long-term, disease-modifying option for IgAN, stopping the disease at its origin. (Barratt J, et al. J Am Soc Nephrol, 2025)
The most recent ORIGIN 3 trial tests the highest atacicept dose used in ORIGIN 2b over 2 years, in a similar group of IgAN patients. This report summarizes the prespecified results from the 36-week interim analysis. This follows the standard template of phase three trials of the initial 9 months of proteinuria allowing accelerated approval, subsequently affirmed by 2-year GFR slope data (seen with targeted release budesonide, iptacopan, atrasentan, sparsentan so far). This has come out of the Kidney Health Initiative (Thompson et al, CJASN 2019) spurred by the ASN partnering with the FDA.
The Study
Methods
Design
Multicenter, randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy and safety of atacicept in patients with IgAN.
Study population
A global study, conducted across 31 countries and 157 investigative sites. Its broad international participation included substantial enrollment from both Asian regions, where IgAN is highly prevalent.
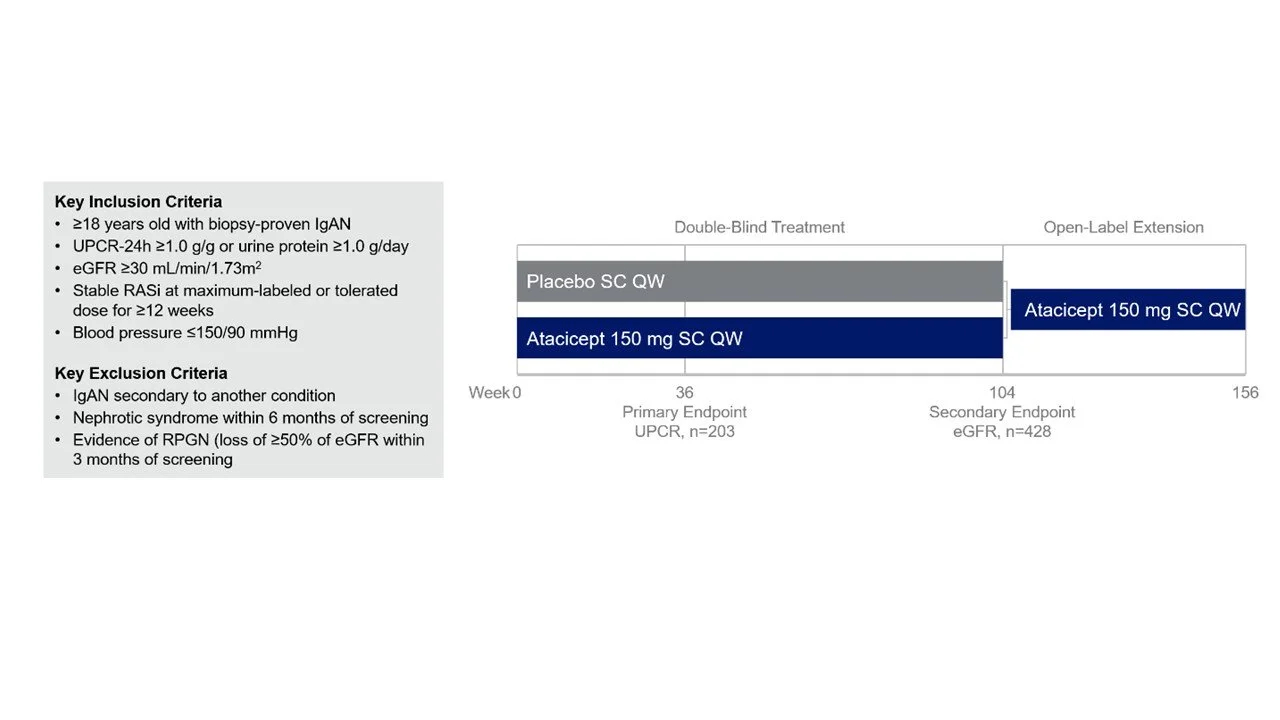
Supplemental Figure 1: Inclusion and Exclusion Criteria and study design from Lafayette R, et al. NEJM. 2025
Notably, the biopsy had to be done within the last 5 years, everyone had to be on a maximally tolerated RASi (flozin use was allowed but not mandated) and GFR had to be > 29 ml/min. Specifically, different phenotypes of IgAN such as nephrotic syndrome and RPGN were excluded. Randomization was stratified by flozin use, GFR subgroups (30- 45, 45 - 90, > 90 ml/min), geographic region (Asia or elsewhere), and proteinuria (> or < 2 g/day).
Intervention
After 4 weeks of screening, participants were randomized to receive weekly subcutaneous injections of 150 mg atacicept a vs placebo. As per protocol:
double-blind treatment: 104 weeks
open-label extension: 52 weeks of atacicept (150 mg)
safety follow-up: 26 weeks after the last dose
Actual interim analysis included week 36 blinded dataset for proteinuria, Gd-IgA1, hematuria, and safety.
Supplementary Image 2: Trial design from Lafayette R, et al. NEJM. 2025 (supplemental protocol)
Outcomes
Primary endpoint:
Change from baseline in urine protein–creatinine ratio (UPCR) based on a 24-h urine sample at week 36
Secondary endpoint (interim):
Change from baseline in the natural log–transformed serum galactose-deficient IgA1 levels Gd-IgA1 levels, hematuria resolution
Statistical analysis
The study planned an interim analysis after the first 203 participants were enrolled, which was the number needed to reliably detect a meaningful difference in proteinuria change from baseline, defined at 33% between atacicept and placebo. Though GFR is measured, GFR slope data is not disclosed until the 104 week follow up for the full study cohort (~ 400 participants) is completed.
Changes in 24-hour UPCR from baseline to week 36 were analyzed using a mixed-model repeated-measures (MMRM) approach. This model included all available data up to the interim cut-off and accounted for the treatment group, baseline UPCR, time, and the stratification factors used during randomization (eGFR category, SGLT2 inhibitor use, and region). The results were first calculated on the natural log scale and then converted back into geometric percentage changes for easier interpretation.
Missing values after treatment discontinuation were handled using a jump-to-reference multiple-imputation method, where missing atacicept values were replaced using expected placebo values. The final treatment effect was expressed as the difference in geometric least-squares means between atacicept and placebo at week 36. Secondary outcomes such as galactose-deficient IgA1, albumin-to-creatinine ratio, and immunoglobulins used similar models, while hematuria was analyzed using a repeated-measures logistic-regression model. No correction was made for multiple comparisons in secondary analyses.
Funding
The trial was funded by Vera Therapeutics, the developer of atacicept. The sponsor was involved in the study design, data collection, and analysis. Several authors were employees of the company and took part in the writing and review of the manuscript, although an independent data monitoring committee oversaw trial conduct.
Results
A total of 203 participants were included in the 36-week interim efficacy analysis, with 106 patients receiving atacicept and 97 patients receiving placebo. For safety, the full cohort of 428 patients were evaluated in total, split evenly between atacicept and placebo (214 in each group).
Figure1: Screening, randomization and follow up, from Lafayette R, et al. NEJM. 2025
Demographics
The baseline demographic and clinical characteristics are seen in Table 1. Almost all patients (99.5%) were already receiving the maximum tolerated dose of a RAS inhibitor at baseline, and just over half of the cohort (53.2%) were flozinated (59 in the atacicept group and 49 in the placebo group). Most participants were Asian or White. About 60% had 1+ or more hematuria, with a median proteinuria just under 2 g/day, and a preserved GFR in mid-60s. The median time from biopsy was ~ 2.5 years with a large spread.
Table 1: Baseline characteristics Lafayette R, et al. NEJM. 2025
Primary Efficacy Endpoint
At week 36, atacicept produced a substantial reduction in proteinuria. The 24-hour urinary protein-to-creatinine ratio decreased by 45.7% in the atacicept group, compared with 6.8% in the placebo group. This corresponded to a 41.8-percentage-point geometric mean treatment difference (95% CI, 28.9–52.3; P<0.001), clearly favoring atacicept. The decline in proteinuria was already evident by week 12 and continued through week 36. Importantly, prespecified subgroup analyses showed that this benefit was consistent across all evaluated categories, including age, sex, region, race, baseline UPCR, baseline eGFR, and SGLT2 inhibitor use.
Figure 3: Subgroup Analyses of the Primary End Point from Lafayette R, et al. NEJM. 2025
Key Secondary Endpoints
Atacicept produced a marked reduction in galactose-deficient IgA1, a central pathogenic biomarker in IgAN. Levels fell by 68.3% at week 36 compared with 2.9% in the placebo group, with reductions visible as early as week 4. Hematuria also improved significantly. Among patients with baseline ≥1+ hematuria, 81% (51 of 63) in the atacicept group achieved resolution versus 20.7% (12 of 58) in the placebo arm. Albuminuria changes mirrored these findings: the urinary albumin-to-creatinine ratio fell by 47.3% in those receiving atacicept compared with 8.8% with placebo.
Figure 2: Percentage change from baseline in key endpoints from Lafayette R, et al. NEJM 2025
Treatment-Emergent Adverse Events (TEAEs)
Treatment-emergent adverse events (TEAEs) were common in both groups but were mostly mild. TEAEs occurred in 59.3% of patients receiving atacicept and 50.0% of those on placebo. As expected for a weekly injectable therapy, injection-site reactions were more frequent with atacicept (19.2% vs 1.9%), and were generally mild. Interestingly, serious TEAEs were actually less frequent with atacicept, occurring in only 0.5% of patients compared with 5.1% in the placebo arm. The single serious event in the atacicept group (a case of cholecystitis) was considered unrelated to the study drug. No deaths were reported. Rates of infections were similar across groups, and importantly, no opportunistic infections and no cases of hypogammaglobulinemia were observed despite expected reductions in IgG, IgA, and IgM levels. Hypersensitivity reactions were noted in 3.7% of atacicept patients compared with 6.5% on placebo.
Discussion
A reduction in proteinuria has now been established as a reliable surrogate endpoint for long-term renal outcomes in patients with IgAN; treatments that decrease proteinuria are generally associated with a slower rate of eGFR decline. This interim analysis of atacicept produced a clear and consistent reduction in proteinuria, along with strong improvements in key biomarkers of IgAN. The magnitude of the proteinuria reduction (−46% vs −7%) and the parallel drop in galactose-deficient IgA1 support the idea that atacicept is acting directly on the upstream pathophysiology of the disease, rather than through non-specific hemodynamic effects. The improvement in hematuria and albuminuria further suggests a meaningful reduction in glomerular inflammation. The emerging evidence from APRIL/BAFF–axis trials including VISIONARY (sibeprenlimab) and ORIGIN-3 (atacicept) highlights how central this pathway is to the biology of IgAN. APRIL drives class switching toward IgA and fuels the overproduction of galactose-deficient IgA1, while BAFF promotes the survival and expansion of mucosal B cells and long-lived plasma cells that sustain pathogenic IgA responses. In VISIONARY, APRIL-selective B-cell inhibition produced robust reductions in proteinuria and IgA-related biomarkers, confirming APRIL activity as a key pathogenic role. ORIGIN-3 goes a step further: atacicept delivers dual BAFF + APRIL blockade, targeting earlier B-cell checkpoints as well, potentially dismantling both the production and maintenance arms of the aberrant IgA response. This broader immunologic effect may explain atacicept’s deep Gd-IgA1 suppression and consistent signal across subgroups.
As newer trials such as APPLAUSE-IgAN (iptacopan, Perkovic et al NEJM 2024) and NEFIGARD (budesonide, Lafayette et al Lancet 2023) continue to illuminate other parts of the cascade, the APRIL–BAFF pathway stands out as the most direct attempt to target the source of pathogenic IgA production. Whether dual pathway inhibition (ORIGIN-3, but also ongoing trials with Telitacicept and Povetacicept) offers a longer-term advantage over APRIL-selective therapy (VISIONARY with sibeprenlimab Perkovic et al NEJM 2025, BEYOND with zigakibart, Kooienga et al Kidney Int 2025) remains a key question that only eGFR slope and renal outcome data will answer. Notably the APRIL-selective therapy also prevents its action at other receptors (BCMA) unlike the -tacicepts.
Among trials in IgAN, the VISIONARY trial (Perkovic V, et al, NEJM, 2025) screened 437 patients and randomized 289, creating a solid mid-sized IgAN cohort. Enrollment came from multiple international sites similar to ORIGIN-3. Both VISIONARY and ORIGIN-3 reinforce the growing role of upstream immune modulation in IgAN. Although sibeprenlimab targets APRIL alone while atacicept blocks both BAFF and APRIL, the two trials showed broadly similar clinical effects: meaningful reductions in proteinuria, strong improvements in key biomarkers, and high rates of hematuria resolution, all on top of contemporary standard-of-care therapy. ORIGIN-3 included patients with slightly higher baseline proteinuria, yet the antiproteinuric effect of atacicept remained consistent across all subgroups, including those on SGLT2 inhibitors.
Safety outcomes were also aligned between trials. Both treatments were well tolerated, with low rates of serious adverse events and predictable immunoglobulin reductions that stabilized without causing hypogammaglobulinemia or opportunistic infections. Mild injection-site reactions and infections were the main differences, but neither raised major safety concerns.
The major limitation shared by both studies is the absence of eGFR slope data. While the early surrogate endpoints are promising, the long-term renal benefits remain unknown (though the phase 2b results are promising). Overall, these interim results suggest that APRIL-based and BAFF/APRIL–based therapies may represent important future disease-modifying options in IgAN, pending a full throated, “Hallelujah” until eGFR data is finalized.
One of the strengths of the trial is that patients were already receiving standard of care treatment: nearly all were on maximal RAS blockade, and more than half were on an SGLT2 inhibitor. This is in contrast to NEFIGARD (Lafayette et al Lancet 2023) where almost no one was flozinated - and merely reflects the evolution of the evidence of flozination and the growing access. The fact that atacicept still produced substantial additional benefit in this optimized background makes the findings highly relevant to current clinical practice. The consistent effect across all subgroups including those with lower or higher eGFR, different geographic regions, and across SGLT2 inhibitor users adds confidence in the robustness of the proteinuria effect.
The safety profile of this study was highly reassuring. Most adverse events were mild, and serious events were actually less common with atacicept than with placebo. Although immunoglobulin levels decreased as expected with BAFF/APRIL inhibition, no cases of hypogammaglobulinemia or opportunistic infections were reported. This aligns with prior data showing that atacicept modulates B-cell survival signals without causing broad immunosuppression.
The major limitation of this interim report is the lack of eGFR data. Because the blinded treatment period is ongoing, kidney function outcomes including the key measure of eGFR slope will only be available at a later date. While the phase 2b results suggested stabilization of eGFR over nearly two years, the long-term benefit in a phase 3 population remains to be seen. Lastly, though this is an exciting time with the FDA approving new therapies for IgAN every few weeks - they are mostly not available or accessible outside a few select countries - and especially in places like Asia where the bulk of IgAN patients live.
Conclusion
Atacicept, a BAFF/APRIL inhibitor, decreases proteinuria, hematuria and Gd-IgA in a worldwide population with mild to moderate risk IgAN. Atacicept appears to be safe, and further data on eGFR preservation is highly anticipated.
Summary by
Sridatta Pawar
Consultant Nephrologist NH
Summary Reviewed by
Brian Rifkin, Cristina Popa,
Sayali Thakare, Swapnil Hiremath