#NephJC Chat
Tuesday Oct 26 at 9 pm Eastern
Wednesday Oct 27 at 9 pm IST
N Engl J Med. 2021 Sep 16;385(12):1067-1077.
DOI: 10.1056/NEJMoa2105675. Epub 2021 Aug 29.
Effect of Salt Substitution on Cardiovascular Events and Death
Bruce Neal, Yangfeng Wu, Xiangxian Feng, Ruijuan Zhang, Yuhong Zhang, Jingpu Shi, Jianxin Zhang, Maoyi Tian, Liping Huang, Zhifang Li, Yan Yu, Yi Zhao, Bo Zhou, Jixin Sun, Yishu Liu, Xuejun Yin, Zhixin Hao, Jie Yu, Ka-Chun Li, Xinyi Zhang, Peifen Duan, Faxuan Wang, Bing Ma, Weiwei Shi, Gian Luca Di Tanna, Sandrine Stepien, Sana Shan, Sallie-Anne Pearson, Nicole Li, Lijing L Yan, Darwin Labarthe, Paul Elliott
PMID: 34459569
Introduction
Sodium is the sixth most abundant element in the Earth’s crust and it’s water soluble salts leach into the ocean making sodium the third most abundant element (following hydrogen and oxygen) in the ocean. Since life arose in the ocean, life evolved with cells bathed in saline solutions. Sodium intake among people varies greatly with the Yanomami tribe (Oliver et al, Circulation, 1975) consuming nearly no sodium, Americans averaging 3400 mg (147 mEq) daily, and Northern Chinese averaging 11,200 mg (487 mEq). Unlike the iguana, we can’t sneeze it out, so we depend on our kidneys to clear dietary sodium and they do this task well however…it is clear from multiple epidemiologic and interventional studies that increased sodium intake increases blood pressure.
High levels of sodium intake, consumed as sodium chloride, is one of the major public health problems around the world. By estimating sodium intake, mostly as 24-hour urinary sodium (with some extrapolation from spot samples, which may be of questionable accuracy) in multiple cohorts most adults consume more than >100 mmol of sodium per day and often over 200 mmol/day in some Asian countries (Brown et al., Int J Epidemiol. 2009). Importantly, this systematic review demonstrates that the source of salt varies across the world (Bhat S, et al., Adv Nutr. 2020). For instance, in China, sodium intake is dominated by salt added in cooking and at the table but this is not true for North America, and Australia.
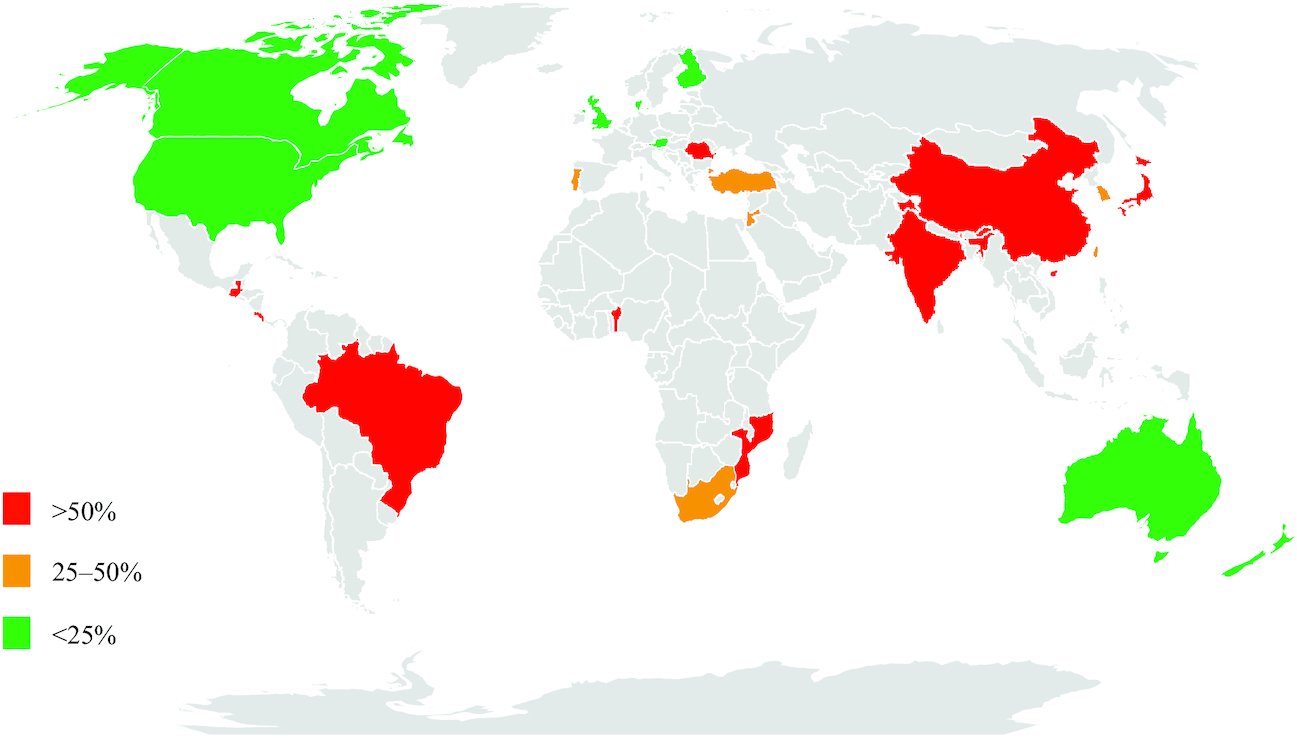
Figure from Bhat et al, Adv Nutr 2020
Contribution of discretionary sources to total dietary salt intake around the globe. Green, <25% of dietary salt from discretionary sources; amber, 25–50% of dietary salt from discretionary sources; red, >50% of dietary salt from discretionary sources. No published data were available for countries shaded in gray.
Previous systematic reviews and meta-analyses have reported the effect of lower sodium intake on blood pressure. A WHO meta-analysis, including randomized controlled trials and cohort studies, reported that blood pressure (BP) fell 3.5/1.8 mmHg when sodium intake was <2 g/day versus ≥2 g/day; however, they were unable to evaluate the effects of reduced sodium intake on clinical outcomes and mortality (Aburto NJ, et al., BMJ. 2013). Another meta-analysis, including 133 RCTs and 12,197 participants, reported a dose-response relationship between reduction in sodium intake and blood pressure. They showed that every 50 mmol (~ 1.15 gram of sodium) reduction in 24-hour sodium excretion was associated with a 1.1/0.3 mmHg reduction in BP (Huang L, et al, BMJ. 2020) So, sodium reduction reduces BP, but we are uncertain about downstream clinical outcomes, since the mean change in BP is quite small.
What about potassium? It is right behind sodium as the 7th most abundant element in the crust and right below it in the periodic table. Potassium is less common in the ocean compared to sodium. The major significance of sodium and potassium, of course, is that they are very similar chemically (see periodic table), and one is the dominant extracellular cation and the other, the dominant intracellular cation. So what is potassium’s role in BP? Apart from possible effects on the vasculature, potassium acts as a sodium switch promoting natriuresis. See this tweetorial from Prof Welling to understand the elegant physiology behind this. Prior work has shown that increased dietary potassium lowers BP and decreases some clinical outcomes, including stroke (Aburto et al, BMJ 2013).
A critical issue is implementing these changes. Most trials of sodium reduction are ‘feeding’ trials, e.g. in the DASH trial, the participants received their breakfast and lunch at the study centers, and were given a packed supper (Sacks et al, NEJM 2001; Ruzicka et al, J Hypertension 2014). One can do this for 8 weeks in a clinical trial, but how do we do this in real life? Would there be a simple, feasible way of coupling the sodium reduction with potassium increase?
The Salt Substitute and Stroke Study (SSaSS) aimed to do exactly this. The investigators randomized high risk people to regular salt or a salt substitute (a composition of 75% sodium chloride and 25% potassium chloride). They then compared the rates of stroke, major cardiovascular events, and death from any cause.
The Study
Study Design
An open-label, cluster-randomized trial, which enrolled 20,995 people from 600 villages in rural China.
Interventions
Use of a salt substitute (75% sodium chloride and 25% potassium chloride) compared to use of regular salt (100% sodium chloride).
Participants in the intervention group were instructed to use a salt substitute (75% sodium chloride and 25% potassium chloride) at an average of approximately 20 g per person per day for all household cooking, seasoning, and food preservation. They were also advised to use the salt substitute less frequently than they had formerly used regular salt. In contrast, those in the control group continued to use regular salt (100% sodium chloride) normally.
Study population
The unit of cluster-randomization: village
The selection process of clusters:
Step 1: A total of 600 villages in five provinces (Hebei, Liaoning, Ningxia, Shanxi, and Shaanxi) were chosen based on established collaborations with local academic and government institutions.
Step 2: Two counties in each province were selected based on their willingness to study participation, their accessibility to the local research team, and their being representative of the level of socio-economic development in the area.
Step 3: Sixty villages in each of 10 counties were selected to recruit approximately 35 persons from each village and to follow them for 5 years.
Inclusion:
Adult men and women who had a history of stroke or ≥60 years of age
Those who had poorly controlled blood pressure
Systolic blood pressure ≥140 mmHg if receiving anti-hypertensive medication
≥160 mmHg if not
Exclusion:
Use a potassium-sparing diuretic, a potassium supplement, or known serious kidney disease (definition not specified).
People unlikely to live longer than 6 months.
People who ate most meals outside of the home.
Outcomes
Primary outcome: Stroke, defined as an acute disturbance of focal neurologic function resulting in death or symptoms lasting more than 24 hours.
Secondary outcomes:
Major adverse cardiovascular events (a composite of nonfatal stroke, nonfatal acute coronary syndrome, or death from vascular causes).
Death from any cause.
Safety outcomes:
Hyperkalemia, defined as either a serum potassium concentration greater than 6.0 mmol/l or a serum potassium concentration greater than 5.5 mmol/l with evidence of typical electrocardiogram changes. Note that hyperkalemia was not measured in everyone. It was on the basis of ‘clinical’ hyperkalemia if a participant presented to a hospital, or cases of sudden death. This was adjudicated by a committee after the fact.
Definite hyperkalemia: Elevated serum K>5.5mmol/L and typical ECG changes documented in medical notes.
Probable hyperkalemia: Elevated serum K>5.5mmol/L or typical ECG changes documented in medical notes.
Possible hyperkalemia: Self-reported serum K>5.5mmol/L or ECG changes but no supporting documentation to verify.
Pre-planned subgroups
Age (>65 vs. ≤65)
Sex (Female vs. Male)
Education level (Primary school or lower vs. Junior high school or higher)
History of stroke (Yes vs. No)
Diabetes (Yes vs. No)
Hypertension (Yes vs. No)
Use of any antihypertensive medication (Yes vs. No)
Baseline blood pressure fitted continuously and divided at its median.
Body-mass index fitted continuously and divided at its mean.
Statistical analysis
Sample size calculation: They calculated a sample size of 21,000 participants in 600 clusters, which would provide 90% of power to detect a proportional difference of 13% or more in the rate of stroke between the two groups at a two-sided alpha level of 0.05.
Primary analyses: They used a hierarchical Poisson regression model to compare the effects of the salt substitute and regular diet on the primary and secondary outcomes according to the intention-to-treat principle.
They used the Kaplan-Meier method to generate cumulative event curves of trial outcomes.
Sponsorship and Support
This study was sponsored by the George Institute for Global Health, where the analyses were performed.
This study was supported by research grants and an investigator grant from the National Health and Medical Research Council of Australia.
The salt substitute was manufactured following the Chinese national standard and purchased by the investigators from local manufacturers in each province.
Results
A total of 20,996 participants were recruited from 600 villages between April 2014 and January 2015, and 20,995 participants were cluster-randomized to the salt-substitute group (10,504 people in 300 villages) or the regular-salt group (10,491 people in 300 villages).
Figure 1 from Neal et al, NEJM 2021
Vital status at the end of follow-up was determined for all the participants , and nonfatal events were evaluated among the participants for 99,473 of 99,533 person-years (>99.9%). Of note, 91.7% of the participants in the intervention group and 6.4% of those in the control group used a salt substitute at the end of a 5-year follow-up.
‘Table 1” from Supplementary Appendix from Neal et al, NEJM 2021
At baseline, the mean age of the participants was 65 years old, roughly half were female, and 73% had a history of stroke. The mean blood pressure was 154/89 mmHg, and 79% of the participants were on at least one antihypertensive medication (either calcium channel blockers, renin-angiotensin system inhibitors, diuretics, and alpha- or beta-blockers). In terms of education level, 73% of participants had only received primary school (or lower)-level education. About 6% had heard about salt substitution, and 1% used salt substitution.
Effect on BP and sodium/potassium intake
Figure 2 C & D from Neal et al, NEJM 2021
Figure 2 shows the effects of salt substitution on BP and sodium/potassium excretion across the follow-up period. Sodium excretion was reduced by 15.2 mmol (350 mg), and potassium excretion was increased by 20.6 mmol (803 mg) by the use of salt substitute. Note the columns: not everyone had 24 hour urine measured, this was in a varying subset only.
Figure 2 A & B from Neal et al, NEJM 2021
BP decreased as well by 3.3/0.7 mm Hg. BP was measured in everyone.
Primary outcome
Figure 3A from Neal et al, NEJM 2021
Participants in the salt-substitution group had a significantly lower rate of stroke than those in the regular-salt group, as shown in Figure 3A. The primary outcome occurred in 29.1 events per 1000 person-years in the salt-substitution group versus 33.7 events per 1000 person-years in the regular-salt group. The rate ratio (RR) was 0.86 [95% confidence intervals (CIs), 0.77 to 0.96].
Subgroup analysis
Figure 4 from Neal et al, NEJM 2021
Prespecified subgroup analysis for age, sex, education level, body-mass index, cardiovascular disease risk factors, or use of antihypertensive medications also showed consistent benefit with the salt-substitute group than in the regular-salt group (see Figure 4).
Secondary outcomes
Figure 3B from Neal et al, NEJM 2021
The rate of major adverse cardiovascular events was lower in the salt-substitution group (49.1 events per 1000 person-years) than in the regular-salt group (56.3 events per 1000 person-years) with a RR of 0.87 [95% CI, 0.80 to 0.94]. However, the rate of nonfatal stroke was not significantly different between the groups (See Figure 3B).
Figure 3C from Neal et al, NEJM 2021
The salt substitute was also shown to be protective against all-cause mortality with 39.3 events in the salt-substitution group versus 44.6 events per 1000 person-years in the regular-salt group (RR, 0.88; 95% CIs, 0.82 to 0.95), as shown in Figure 3C.
Adverse events
Figure 3D from Neal et al, NEJM 2021
There was no difference in clinical hyperkalemia, including participants who had definite, probable, and possible hyperkalemia, between the groups with a RR of 1.04 [95% CIs, 0.80 to 1.37], as shown in Figure 3D. As above, note that serum potassium was not routinely measured.
Discussion
The SSaSS is an open-label, cluster-randomized trial, involving persons who had a history of stroke or were 60+ years of age with high blood pressure, and showed that those who used a salt substitute had lower rates of stroke, major adverse cardiovascular events, and death from any cause than those who continued to use regular salt. This study also did not find an increased risk of hyperkalemia in those assigned to the salt-substitution group.
Strengths
Randomized design.
Easy to implement intervention
Large effect sizes on trial outcomes.
Long follow-up compared with previous studies.
Limitations
Incomplete adherence to the use of the salt substitute. (e.g., the possibility of consumption of regular salt outside the home; 6.4% of participants who were assigned to the regular-salt group reported using a salt substitute.)
Elevated potassium levels might have not been captured correctly.
The biological gradient was not evaluated.
Open label design
Implications on the public health
The incidence of stroke could be reduced by the population-wide use of a salt substitute.
Because of its low cost, a salt substitute may contribute to reducing the health inequities related to cardiovascular disease.
This can settle the voices which have critiqued the lack of clinical outcomes from sodium reduction from previous trials. However, can we say the benefit was from a decrease in sodium (~ 15 mmol/day) or an increase in potassium (~ 20 mmol/day)? From a generalizability or implementation perspective, this is quite important. It is not reducing salt in diet that improves outcomes, it is substituting the salt with a K-enriched salt that is beneficial. Secondly, this was a population in whom most of the added sodium is in the household while cooking. So this intervention would not be as effective in places where most of the salt consumed is not from the salt shaker at the table, namely most of the western world (Bhat S, et al., Adv Nutr. 2020). For these places, other options would have to be considered: changing sodium content in the food supply and mixing more potassium. This study excluded potential participants for ‘serious renal impairment’ which itself was undefined. That does open up another question, since an intervention like that would affect the entire food supply, with no easy way to exclude people living with kidney disease, who might be at higher risk of hyperkalemia.