#NephJC Chat
Tuesday July 26 9 pm Eastern
Wednesday July 27 9 pm IST
Lancet 2022 Apr 9;399(10333):1391-1400. doi: 10.1016/S0140-6736(22)00369-5
Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomised, controlled trial
Justin A Ezekowitz, Eloisa Colin-Ramirez, Heather Ross, Jorge Escobedo, Peter Macdonald, Richard Troughton, Clara Saldarriaga, Wendimagegn Alemayehu, Finlay A McAlister, JoAnne Arcand, John Atherton, Robert Doughty, Milan Gupta, Jonathan Howlett, Shahin Jaffer, Andrea Lavoie, Mayanna Lund, Thomas Marwick, Robert McKelvie, Gordon Moe, A Shekhar Pandey, Liane Porepa, Miroslaw Rajda, Haunnah Rheault, Jitendra Singh, Mustafa Toma, Sean Virani, Shelley Zieroth, SODIUM-HF Investigators
PMID: 35381194
Introduction
Salt is considered a root cause of hypertension - and we have seen recent well done trials reporting that use of a salt substitute (75% sodium chloride with 25% potassium chloride) reduces blood pressure, stroke, and all cause mortality. After stroke, the cardiovascular outcome most closely linked to hypertension is heart failure. So it is surprising that the trials of sodium restriction in heart failure have resulted in inconsistent results. While some studies showed benefits to sodium restriction (SODIUM-HF pilot study. Am Heart J. 2015, Hummel et al. Circ Heart Fail. 2013) and worse outcomes with increased sodium intake (Arcand et al. Am J Clin Nutr. 2011), other studies have found sodium restriction to be harmful (Pasquale et al. J Card Fail. 2009, Paterna et al. Am J Cardiol. 2009, Doukky et al. JACC Heart Fail. 2016). Indeed, a well done systematic review (Mahtani et al, JAMA Intern Med 2018) reported such a high risk of bias and heterogeneity that they deemed a pooled meta analysis inappropriate, and that the way forward was to do a definitive trial. The conflicting results have been attributed to diversity of patients and variable adherence to dietary sodium restriction. Despite the mixed and uncertain evidence, the guidelines recommend sodium restriction. It is so plausible, how can we not?
Table from the SODIUM-HF trial protocol of current heart failure guidelines
Sodium Interventions
One aspect worth noting is that, unlike drug or device interventions, restricting dietary sodium is a lifestyle and behavioral modification. So prescribing sodium restriction is not the same as prescribing a pill. Sodium restriction requires comprehensive and sustained changes in a person’s lifestyle. In the hypertension literature, there have been several efficacy trials of sodium restriction, where food was provided to participants to ensure that the prescribed sodium intake was actually achieved (Ruzicka et al, J Hypertension 2014). However, such trials do not tell us what happens in real-life setting which is demonstrated by effectiveness, or pragmatic trials (see Manasi’s blog on this for NephTrials). The particular trial we are discussing today, is a pragmatic trial. Food was not actually provided to participants. The intervention was based on the results of pilot trial where participants were provided advice, and meal plans to achieve the desired sodium reduction. However, in this trial, as well as the pilot trial, the sodium restriction was assessed with a 3-day food diary (Colin-Ramirez et al, Am Heart J 2015).
Sodium and Salt
Millimols and Milligrams
These are sometimes confusing so here is a simple table to keep things straight. The conversion depends on the molecular weight - which for sodium (Na) is 23 g/mol. Salt (NaCl) has a molecular weight of 58.5 g/mol.
1 mole (mol) = 23 gram (g) sodium
100 mmol = 2.3 g or 2300 mg sodium
In the trial title and description, though the authors mention 100 mmol/1500 mg sodium - since the baseline was around 100 mmol (2300 mg) it is likely the intervention was aimed at 65 mmol (1500 mg) sodium.
The study
Design
This was a multicenter, open-label, pragmatic, randomized controlled trial. It was conducted at 26 centers across 6 countries.
Inclusion Criteria
The inclusion criteria were
Age ≥ 18 years
with
Chronic heart failure (defined as NYHA functional class 2–3)
and
Receiving optimally tolerated guideline-directed medical therapy (i.e. beta-blockade, RAS blockade including ARNI, and MR antagonists; flozins were not part of GDMT when the trial was designed)
The diagnosis of heart failure was based on local clinical guidelines and expert clinician opinion, and inclusion and exclusion criteria did not include a particular target for ejection fraction or natriuretic peptide.
Exclusion Criteria
The exclusion criteria were:
Average dietary sodium intake of less than 1500 mg/day, based on food diary, not 24 hour urinary sodium
Serum sodium concentration of less than 130 mmol/L
Estimated glomerular filtration rate (eGFR) of less than 20 mL/min per 1.73 m² or haemodialysis-dependent kidney disease
Admission to hospital for a cardiovascular cause in the past month
Intervention
Participants were randomly allocated in a 1:1 manner to either usual care or to a low sodium diet.
The low sodium diet group was assigned a target sodium intake of less than 65 (not 100 as stated) mmol/day (1500 mg Na/day). The participants were provided with meal plans, tested and locally adapted from the pilot study. The diet plans were prescribed to meet the caloric requirements, consistent with cardiovascular diet guidelines. They also received behavioral counseling by trained professionals at each clinic visit (details below). Notably the trial had a ‘Dietitians Working Group’ - some of whom are in the authors list as well (see page 2-4 of supplement for full list). The dietitians also made phone visits more often to the intervention group. See Figure 1.
The control group was given general advice to restrict dietary sodium, as provided during routine clinical practice.
There was no run-in period or specific fluid restriction or dietary supplementation recommended.
The total intervention period was 12 months and participants were followed for an additional 12 months thereafter. The control group had clinical visits at baseline, at 6 and 12 months, while the intervention group had those visits plus two extra visits at 3 and 9 months.
At each follow-up visit, bodyweight, 3-day food records, and NYHA functional class were assessed, and participants were asked to complete quality-of-life assessments (Kansas City Cardiomyopathy Questionnaire, KCCQ). See Image 1 for a schematic of the study.
Image 1: Study schema.
Outcomes
The primary outcome was a composite of cardiovascular (CV) related hospitalization, emergency department visits, and all-cause mortality within 12 months of randomization. CV-related events were not restricted to hospitalization for heart failure (HHF) but also for acute myocardial infarction (MI), resuscitated sudden death, and other CV causes. Though the trial was open label, there was a blinded Clinical Events Committee which adjudicated all these events.
Secondary endpoints included the time to first event within 12 months; change in quality of life score, physical limitation score; change in 6-min walk distance; and change in NYHA functional class.
Analytic plan and sample size
Based on an “assumed” event-rate of 25% in the usual care group, it was estimated that a sample size of 992 patients would give a reasonable power (80%) to detect a 30% reduction in primary outcome in the low sodium diet group. However, the trial had to be stopped early, as advised by the Data Review Committee upon review of the data from the first 500 participants with complete 12-month follow up, operational feasibility, and the effect of Covid-19 pandemic.
The outcomes were assessed using the intention-to-treat model. The data were adjusted for prespecified baseline patient characteristics (including age, sex, NYHA functional class, calorie intake, sodium intake, left ventricular ejection fraction, body-mass index, and eGFR), using multivariable Cox regression model. Prespecified subgroup analyses of the primary composite outcome was performed on the basis of age (<65 vs ≥65 years), sex, renal function (eGFR <60 vs ≥60 mL/min per 1.73 m²), diabetes, hypertension (yes vs no), and left ventricular ejection fraction (<40% vs ≥40%). Additional post-hoc sensitivity analyses were also performed to assess the risk of the primary outcome by strata of baseline dietary sodium intake (≤1500, 1501–3000, and >3000 mg/day) and the effect of the intervention on patients across these tertiles, and by baseline use of a renin angiotensin system inhibitor and geographical region. All the analyses were done using SAS software (version 9.4).
Funding
The study was funded by Canadian Institutes of Health Research and the University Hospital Foundation, Edmonton, Alberta, Canada, and Health Research Council of New Zealand. They did not have any role in study design, data collection, data analysis, data interpretation, or writing of the article.
Results
Baseline characteristics
A total of 841 patients were randomized between March 24, 2014, and Dec 9, 2020. This was less than the initial planned sample size - as on the advice of the data review committee when a planned interim analysis when the initial 500 enrolled patients had completed the 12 month follow up. Apparently this was on the basis of futility - with feasibility and COVID also being mentioned, in Dec 2020 - and then 12 months follow up was collected for the last few hundred enrolled patients to wrap up the trial in Dec 2021. Interestingly, the number of participants screened and eligible is not provided like it should be as specified in CONSORT (Figure 1).
Figure 1. Trial Profile, from Ezekowitz et al, Lancet 2022.
Out of the total randomized patients, 806 were included on an intention to treat basis (after 35 patients were already excluded post-randomisation for an unknown “technical error”). Baseline characteristics are shown in Table 1. More than two-thirds were diagnosed with heart failure more than a year ago, and at least one-third had had a hospitalization for heart failure in the preceding year. A third of the population was female and almost 60% were recruited in Canada.
Table 1. Baseline characters of participants from Ezekowitz et al, Lancet 2022.
Around 80% patients were on renin-angiotensin aldosterone system (RAAS) inhibiting agents (including ACEi/ARBs, ARNIs and MRAs) at the time of randomization. More than 85% of patients were on beta-blockers. Approximately 60% were on MRAs. Use of SGLT2 inhibitors (flozins), or other natriuretic drugs, is not mentioned in the table. Average eGFR for the whole cohort was ~ 60 ml/min per 1.73 m².
Table 1 (cont’d). Baseline characters (labs and medications) of participants, from Ezekowitz et al, Lancet 2022.
Change in sodium intake
The median baseline daily sodium intake was 2286 mg/day for the low sodium diet group and 2119 mg/day for the usual care group. The median sodium intake was 1649 mg/day at 6 months and 1658 mg/day at 12 months (a decrease of approximately 28%) for the low sodium group, while that in the usual care group was 2021 mg/day and 2073 mg/day at 6 and 12 months, respectively (a decrease of approximately 4%). Thus, the achieved median intake was a bit higher than the target (1500 mg or 65 mmol per day) for the intervention. The blood pressure decreased a bit at 3 months in the intervention group (SBP from 118 to 112 mmHg, p=0.14) but subsequently came back up to be similar to that of the control group. No changes were noted in body weight, caloric intake, or potassium intake (stable at ~ 2300 mg or 60 mmol per day).
Figure 2. Changes in sodium intake (A), blood pressure (B), bodyweight (C), and energy intake (D), from Ezekowitz et al, Lancet 2022.
Outcomes
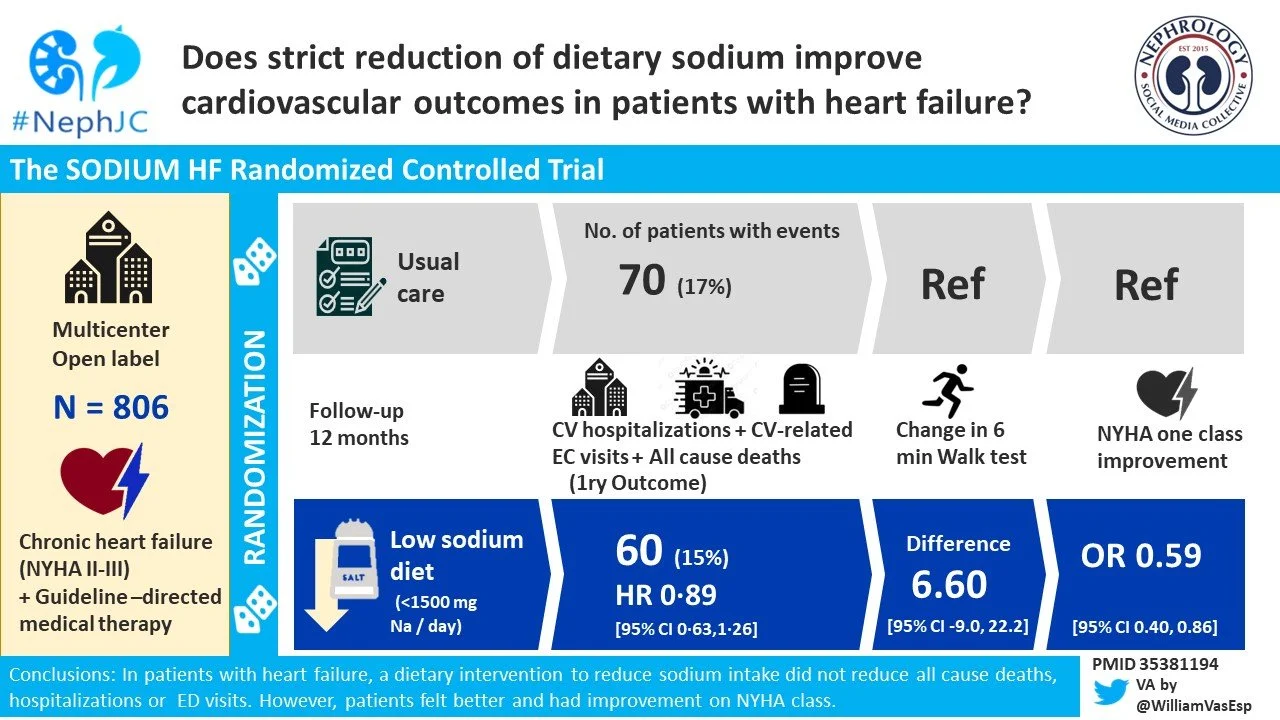
At 12 months, the primary outcome occured in 15% of patients in the low sodium diet group and 17% of patients in the the usual care group (HR 0.89, p=0.53; Figure 3A). The individual components of the composite primary outcome likewise did not differ between the groups (Figure 3B-D).
Figure 3. Composite primary outcome (A) and secondary outcomes of all-cause mortality (B), cardiovascular related hospitalization (C), and cardiovascular-related emergency department visit (D), from Ezekowitz et al, Lancet 2022.
Table 2. Primary and selected secondary outcomes, from Ezekowitz et al, Lancet 2022.
Subgroup Analysis
Among the pre-specified subgroups, the outcomes look fairly consistent, except when stratified by age. In those less than 65 years of age, the intervention group had a lower event rate. See Figure S2 above.
In addition, they also performed a post hoc subgroup, based on baseline sodium intake and RAASi use, which does not show any difference as shown below. Of interest, there is a subgroup with sodium intake of less than 1500 mg, though this level of sodium intake is an exclusion criteria from the study.
Quality of Life
Among other secondary outcomes, improvement in quality of life assessed by KCCQ was noted; mean between-group difference in the overall summary score was 3.38 points (p=0.011), clinical summary score was 3.29 points (p=0.011), and physical limitation score was 3.77 points (p=0.017) (Figure 4). A change of 5, 10, and 20 points in the KCCQ scores represent small, moderate-to-large, and large-to-very large clinical changes, respectively, see Image 2 below.
Image 2 Mean Differences in KCCQ Scores Among Patients With Different Magnitudes of Clinical Change from Spertus et al, JACC 2020.
Figure 4. Change in KCCQ overall summary score (A), and KCCQ physical Limitation Score (B), from Ezekowitz et al, Lancet 2022.
Also, patients in the low sodium diet group had a greater likelihood of improving one NYHA class than usual care group (odds ratio 0.59; p=0.006). On the other hand, no difference in 6-min walk distance at 12 months was seen between the groups. See Figure 4 (cont’d).
Figure 4 (cont’d). Change in 6-min walk test (C), and NYHA functional class (D), from Ezekowitz et al, Lancet 2022.
Discussion
Dietary restriction of sodium has been at the foundation of heart failure management for decades, although there is only low quality evidence to support this practice. In fact, some data showed harm with sodium restriction, which is postulated to be caused by concomitant nutritional deficiencies caused by restricted dietary intake or by reflex RAAS activation stimlated by sodium deficiency. However, the current guidelines continue to recommend restricting sodium intake to < 2300 mg/day.
Image 3. Non-pharmacologic intervention, dietary sodium restriction, from 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
Overall Sodium HF is a well-designed study, considering that diet-based trials are difficult to conduct. The authors employed a pragmatic approach, tested in a pilot study, where meal plans were adapted to regional preferences, while meeting energy requirements. The study protocol also ensured periodic monitoring of dietary adherence by reviewing 3-day food diary.
This trial was able to enroll and randomize more than 800 patients, which is by far the largest number of patients in a trial of this type. However, the trial was stopped prior to enrolling the required 992 patients and had an actual event-rate in the primary group of 17% when the initial assumption was 25%, leaving it under-powered. In addition, the paper fails to provide us with information on how many total number of patients were screened for this study; this information is not likely to influence the results but something that is worth mentioning in terms of generalizability.
The level of dietary sodium restriction that the study was aiming to achieve is also a bit confusing: the title discusses restriction to less than 100 mmol/day, which is equal to 2300 mg/day; the study on the other hand focuses in restricting the intake to 1500 mg/day which is equal to 65 mmol/day. This we believe is an important issue that needs to be clarified by the authors. Since most guidelines currently recommend limiting sodium intake to 2-3 gram/day (which is equal to 100-130 mmol/day), this point has widespread implications as it will help us understand the objectives of study further: whether this study was looking to add evidence in support of the current guidelines or was it looking to enforce stricter sodium restrictions.
Coming to the results, the study did not show any improvement in clinical outcomes with reduction in events, except for improvement in NYHA heart failure class and KCCQ scores. This can be attributed to multiple factors:
The groups started with a comparable baseline median daily dietary sodium intake (2.1 - 2.3 grams/day). At the end of the 12 month period, there was a reasonable separation between the daily sodium intake in both groups (2.07 vs 1.66 grams/day), however the absolute difference in the median sodium intake was only ~ 415 mg/day (or 18 mmol/day). A couple of things need to be considered here. First, the daily sodium intake of both groups is already at par with the current guidelines. So it is unclear if further decrease produces any more reduction in the cardiovascular risk. Secondly, It is unclear if a difference of daily dietary sodium intake of 415 mg/day or 18 mmol/day between the groups is enough to show any benefits. Lastly, is it possible that the actual difference in sodium intake was even smaller since this was measured based on food diary and not a 24 hour urine collection? Note that there was a small BP drop at 3 months but no difference thereafter between groups - and the weight also did not change so there is scant firm physiological evidence of sodium reduction.
Along the same note, the intervention was a pragmatic one based on meal plans and dietary advice and not food provision. Would a more efficacious intervention have had an effect? Note the recently published SSASS trial (NephJC summary) - in which the intervention was a salt substitute and was easier to implement, resulted in a 15 mmol/day decrease in sodium coupled with a 21 mmol/day increase in potassium with an impressive clinical benefit. Could this difference be explained a benefit seen in a different population, a benefit from the potassium increase, or just a benefit from a more effective intervention?
With any open label trial, there is always a risk of introducing bias in the study. Patients in the usual care group might reduce their dietary intake of sodium however this was not evident from the data. The open label design might also explain the improved score on the KCCQ index and improvement in NYHA class which are actually subjective measurements, while on the other hand, the 6-minute walk test, which is an objective assessment, did not show any improvement.
We do not have data on natriuretic usage - loop diuretics, thiazide/thiazide-like diuretics or flozins. It is possible that any benefit from sodium restriction is merely from better volume control - which might just as easily be achieved with these drugs in the control group.
Lastly, one may hypothesize that the benefit was too small to be seen as this was a lower risk population - after all their event rates were lower than expected. Would a larger trial in a higher risk population show a benefit?
Conclusion
With the results of this trial, there’s only one thing that can be said, which is that with a baseline dietary sodium intake of 2000 mg/day, advocating dietary changes to reduce sodium intake further does not result in improvement in clinical outcomes. It would be a stretch to say sodium reduction does not have a benefit - but that sodium reduction implemented as dietary counseling does not have a benefit from the largest trial performed in this population. The time being spent on dietary counseling could perhaps be better spent on prescribing a flozin?