#NephJC Chat
Tuesday May 23 at 9 pm Eastern (AEST = June 8th, 11am)
Wednesday May 24, 2022, at 9 pm Indian Standard Time and 4:30 pm BST (AEST = June 9th, 2am)
Lancet 2023 Mar 31;S0140-6736(23)00569-X. doi: 10.1016/S0140-6736(23)00569-X. Online ahead of print.
Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomized, double-blind, active-controlled clinical trial
Hiddo J L Heerspink, Jai Radhakrishnan, Charles E Alpers, Jonathan Barratt, Stewart Bieler, Ulysses Diva, Jula Inrig, Radko Komers, Alex Mercer, Irene L Noronha, Michelle N Rheault, William Rote, Brad Rovin, Howard Trachtman, Hernán Trimarchi, Muh Geot Wong, Vlado Perkovic; PROTECT Investigators
PMID: 37015244
See also: PROTECT study baseline characteristics
Introduction
In recent years, the world of nephrology has been witnessing the burgeoning landscape of therapeutic clinical trials in IgA nephoipathy (IgAN) (Yan Cui et al, Ren. Fail., 2022). The latest landmark studies published include the TESTING 2.0 trial (Lv J et al, JAMA 2022; reviewed in the NephJC summary), EMPA-KIDNEY trial (Herrington et al, NEJM 2023; NephJC summary) and the NefIgArd trial (Barret et al, KI 2023; NephJC summary). However, definitive treatment for IgAN remains a topic of debate as witnessed in the hotly contested battle in the Nephmadness 2023 bracket of non-immunosuppressive vs immunosuppressive therapy for IgAN. These trials do show that immunosuppression does benefit in certain select subpopulations of IgAN.
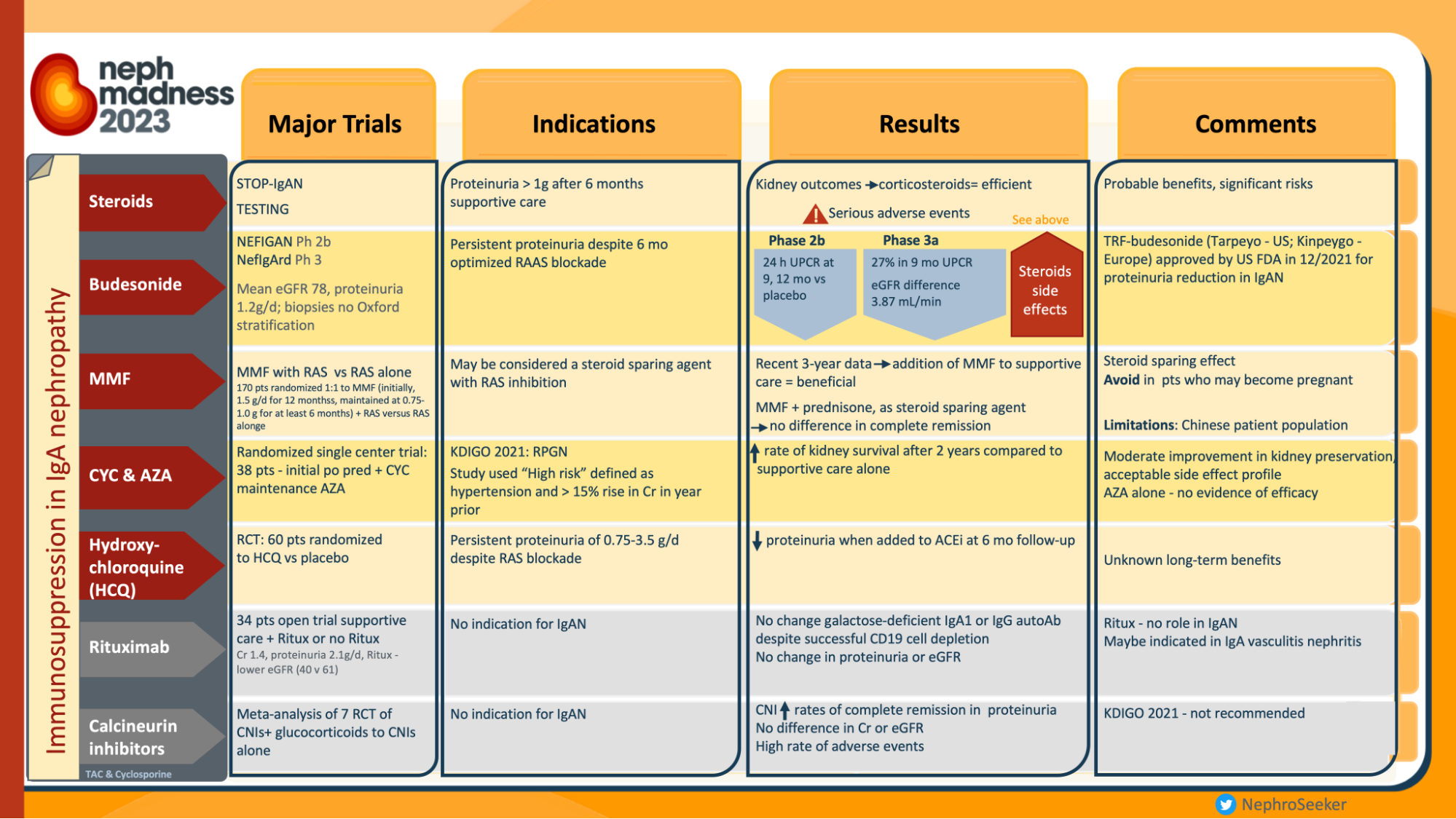
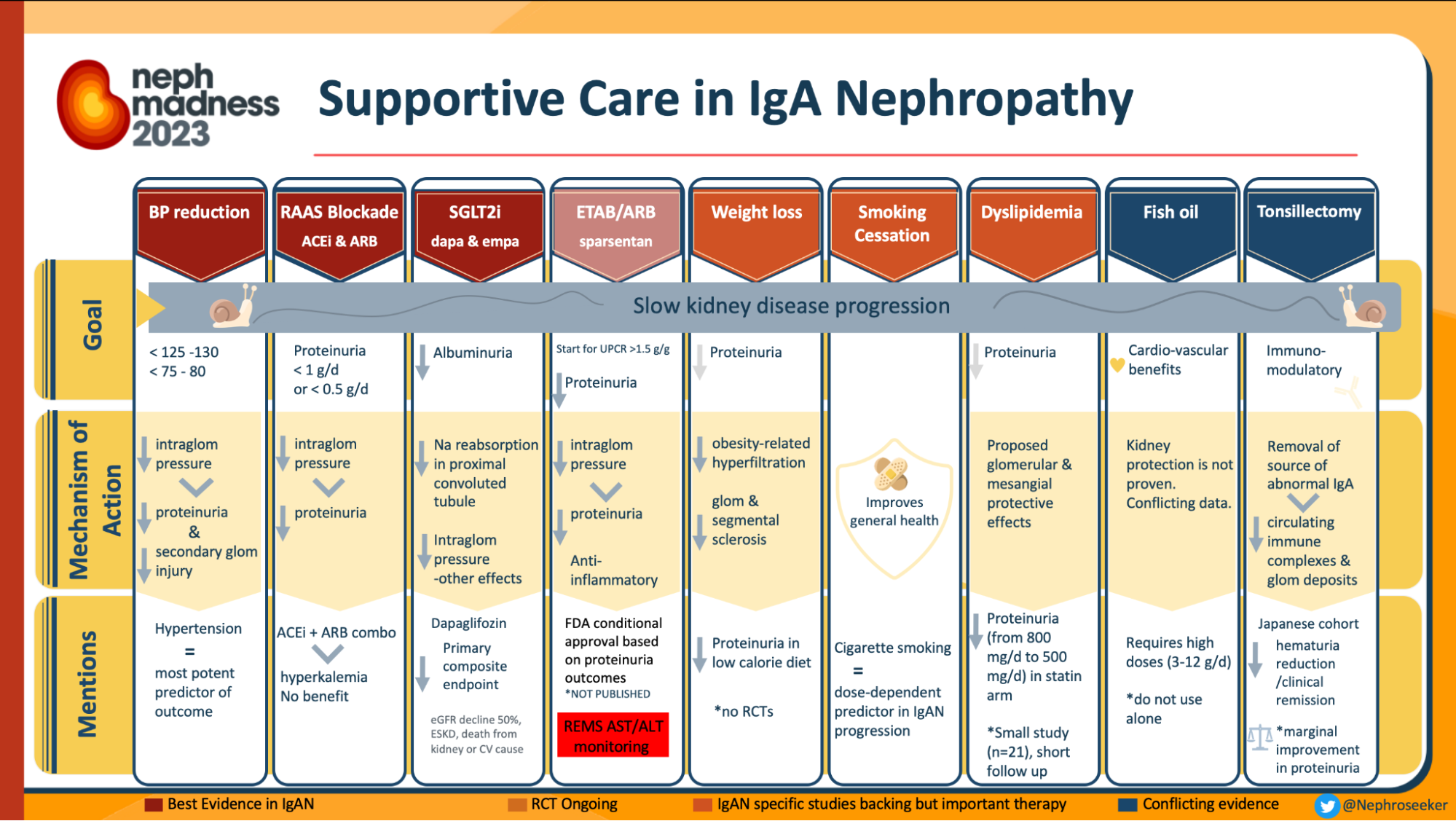
Infographics on treatment in IgAN, from Nephmadness 2023
However, the KDIGO guidelines prioritizes baseline non-immunosuppressive based strategies, to slow the rate of IgAN progression. This is primarily RASi (renin-angiotensin system inhibitors) and now flozins (after DAPA-CKD and EMPAKIDNEY). Is there anything else we can use?
There is: endothelin antagonism. Endothelin (ET) is a 21-amino acid polypeptide produced mainly by endothelial cells, also by cells of the renal system, such as the epithelial and mesangial cells. It is present in three isoforms: ET-1, ET-2 and ET-3 of which ET1 has the greatest vasoconstricting effect. It acts through binding to ETA and ETB (G protein coupled receptors) producing opposite effects in the kidney. In pathological conditions, the hyperglycaemia, acidosis and the presence of insulin, angiotensin II and proinflammatory cytokines causes the increase of ET-1 concentration, which produces deleterious effects on renal function as shown in the figure below, eventually, GFR decline. Therefore, endothelin receptor antagonists (ERAs) have been proposed as a therapeutic strategy to reduce proteinuria and slow the progression of kidney disease.
Scheme of the endothelin system from Martínez-Díaz et al, Int. J. Mol. Sci. 2023
Bringing the promise of no fibrosis, it is no wonder that they were the topic of a multitude of trials, for various indications: PAH, resistant hypertension (NephJC discussion of PRECISION), and FSGS. However, they are not without risks. Atrasentan in diabetic CKD (SONAR trial Heerspink et al, Lancet 2019, NephJC discussion) had a higher risk of fluid retention and CHF. Bosentan and Ambrisentan are used for PAH. Apart from sodium retention and heart failure, hepatotoxicity has been the Achilles’ heel of ERAs. Atrasentan is also being studied in IgAN patients on optimized RASi in the AFFINITY (Phase II) and the ALIGN (Phase III) trial and the interim analysis have some encouraging results.
Chronology of the main discoveries and advances in the endothelin field over the last 30 years from Barton M et al, Hypertension. 2019
Both Angiotensin II (Ang II) and ET-1 are powerful vasoconstrictors and implicated in the pathogenesis of hypertension, cardiovascular and kidney disease. They increase the production and function of one another and act in a positive feedback loop (Komers et al, AJP 2016). Simultaneous antagonism of both the renin-angiotensin system (mediated by angiotensin II via AT1 receptors) & the endothelin system (mediated by ET-1 via ETA receptors) can produce a greater reduction in blood pressure & added CV and renal benefit than antagonizing either system alone. Sparsentan is the drug developed with this purpose. It is the first in class, dual endothelin A/angiotensin 2 receptor blocker (DEARA). It was earlier studied in FSGS in the DUET (Phase II) (Trachtman et al, JASN 2018) and is being studied in DUPLEX (Phase III) studies. DUPLEX has achieved the proteinuria endpoint as per the press release but as per the latest press release the DUPLEX study did not achieve the primary efficacy eGFR slope endpoint over 108 weeks of treatment. More on this topic later.
Has Sparsentan, a DEARA added another feather in the cap of the supportive armamentarium in IgAN? Let us delve more into the interim results of the PROTECT study and find out.
The Study
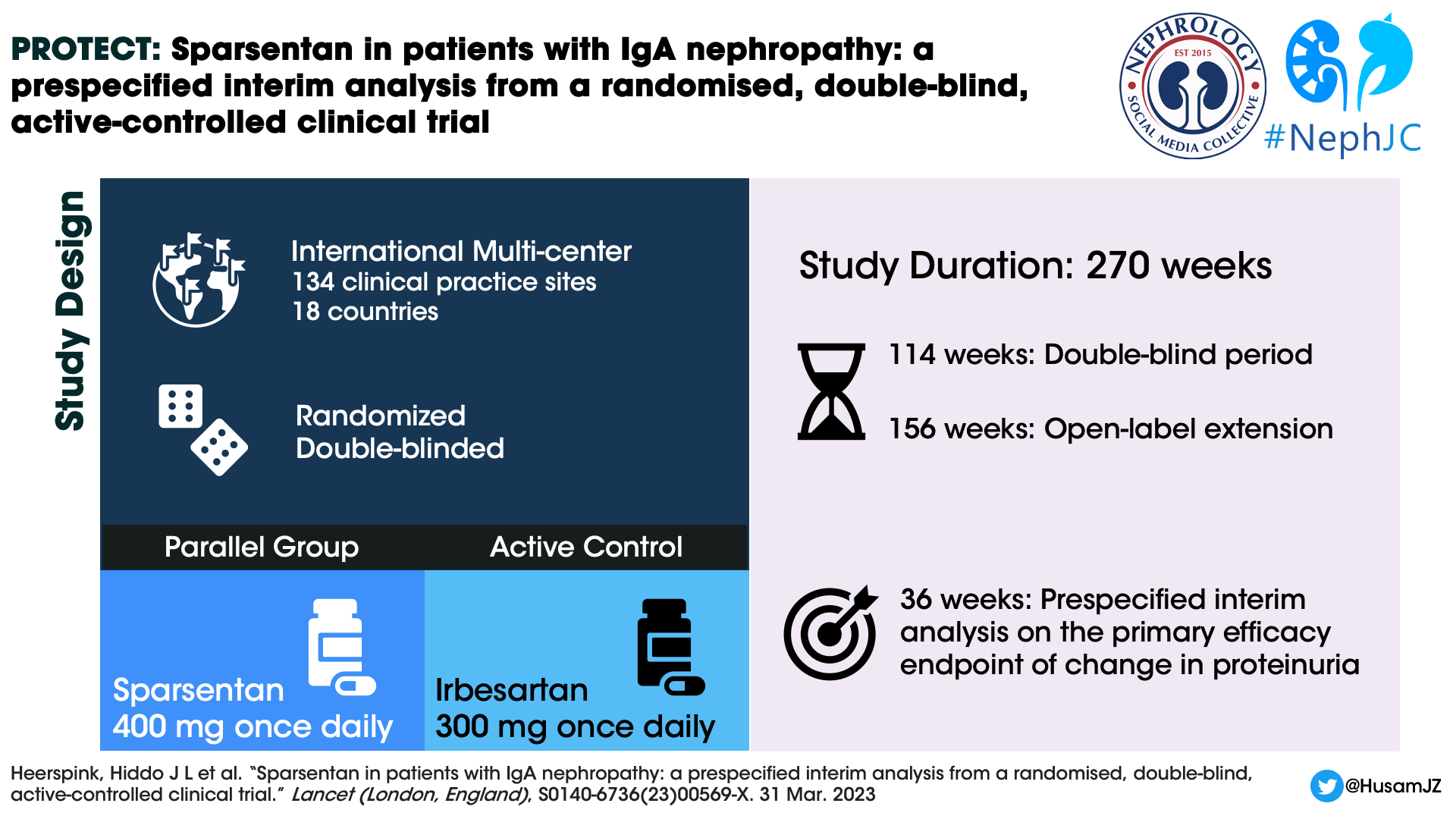
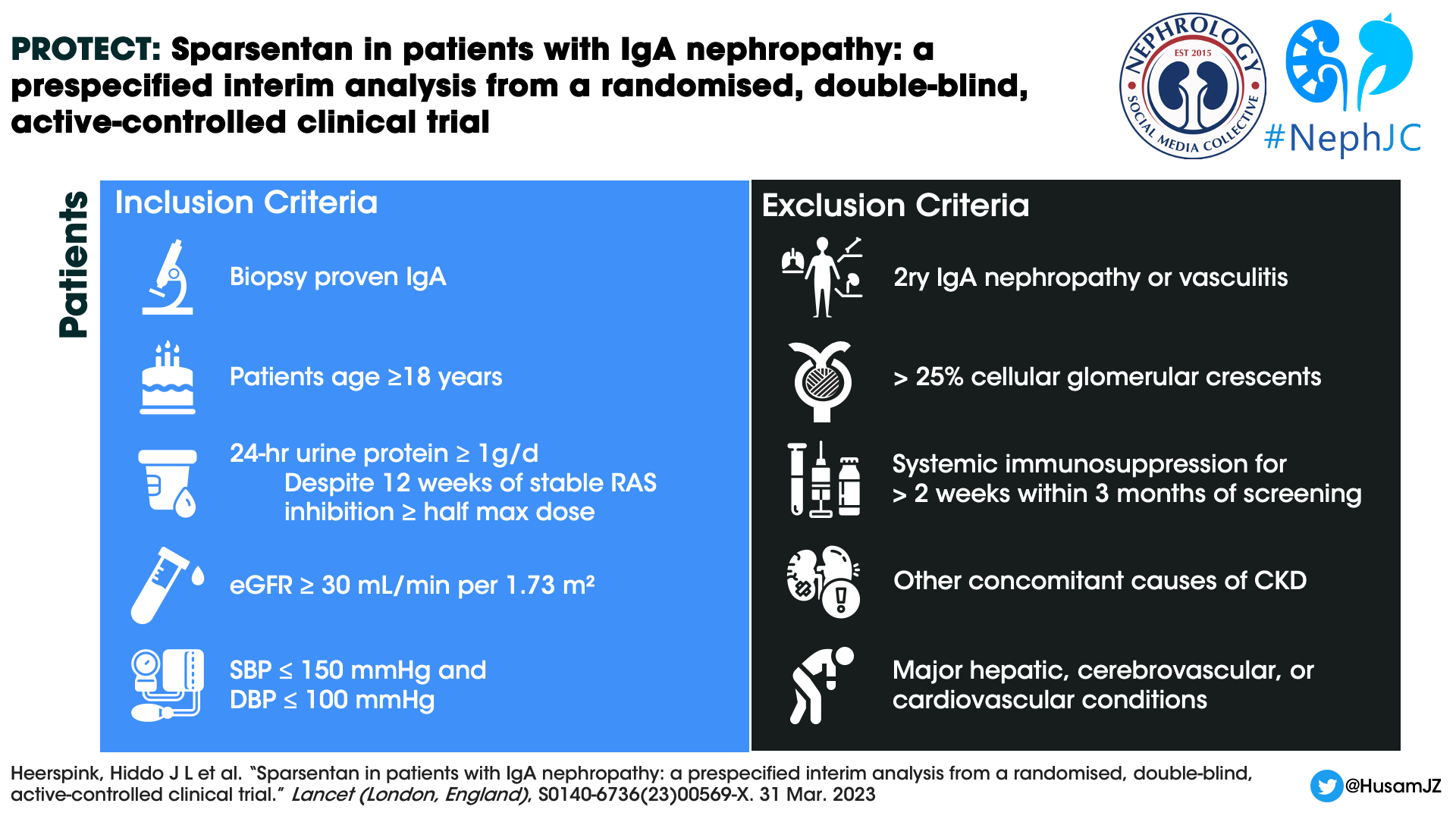
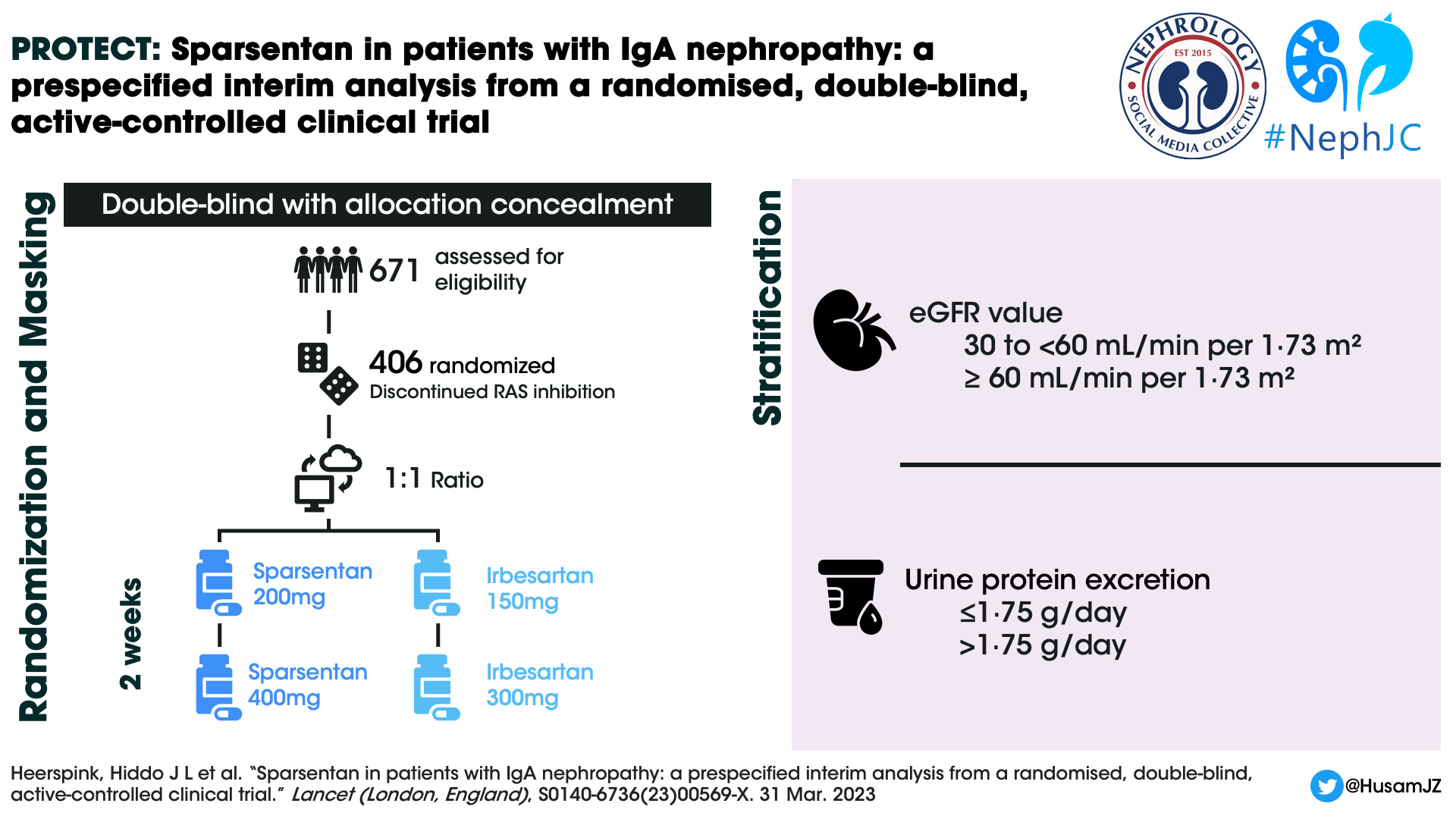

Design
The PROTECT study is an international, multicentre, randomized, double-blind, parallel group, active controlled phase 3 clinical trial. The present report is a 36 week interim analysis mostly on proteinuria reduction.
Study population
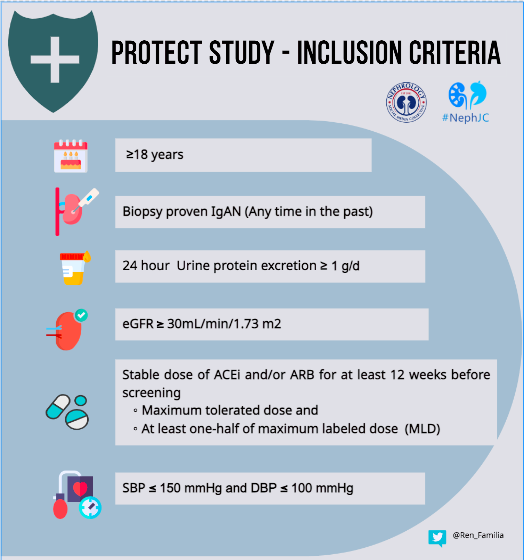
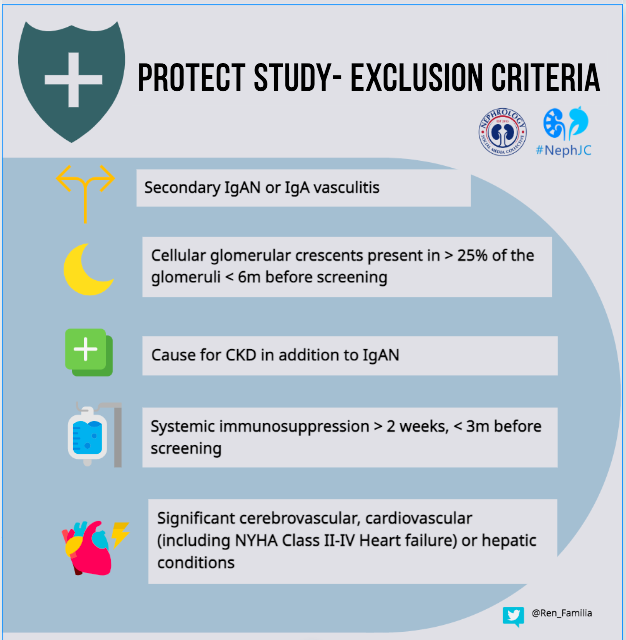
The study is being conducted in 134 clinical practice sites in 18 countries. The baseline characteristics of the patients in the PROTECT study have been mentioned here separately. The key inclusion and exclusion criteria were as shown in the infographic below.
Intervention
Patients were randomized 1:1 to sparsentan or irbesartan group using a predefined computer-generated randomisation schedule and stratified by screening eGFR (30 to < 60mL/min per 1.73m2 and ≥ 60 mL/min per 1.73 m2) and urine protein excretion (≤1.75 g/day and >1.75 g/day). The enrolled patients discontinued RAS inhibition and any other prohibited concomitant medications (SGLT2i included) before the randomisation visit. There have been six global amendments to the original study protocol (April 2018) till date. The sixth global amendment (November 2022) was directed at the open-label extension and added a sparsentan plus SGLT2 inhibitor sub-study to the protocol. The study design is as shown in the figure below.
At the end of week 2, titration to the target dose of 400 mg once daily of sparsentan or 300 mg once daily of irbesartan was done after evaluation of the dose tolerance. This was defined as SBP > 100 mm Hg and DBP > 60mm Hg and no treatment-emergent adverse events (TEAEs), such as worsening edema or laboratory findings (e.g. serum potassium > 5·5 mEq/L). Participants with asymptomatic blood pressure values of 100/60 mm Hg or lower, or with clinical symptoms of orthostatic hypotension, continued the initial dose.
During follow up, dose titrations were permitted at any time at the investigator’s discretion. Also, BP lowering medications could be initiated or adjusted at the discretion of the investigator to reach the guideline-recommended blood pressure target of 125/75 mmHg. Study visits occurred at weeks 2, 4, and 6, followed by week 12 and every 12 weeks thereafter. Proteinuria and albuminuria were assessed by 24-h urine collection at each study visit and analyzed at a central laboratory.
Supplementary Figure 1: Trial Design from Heerspink HJL et al, Lancet, 2023
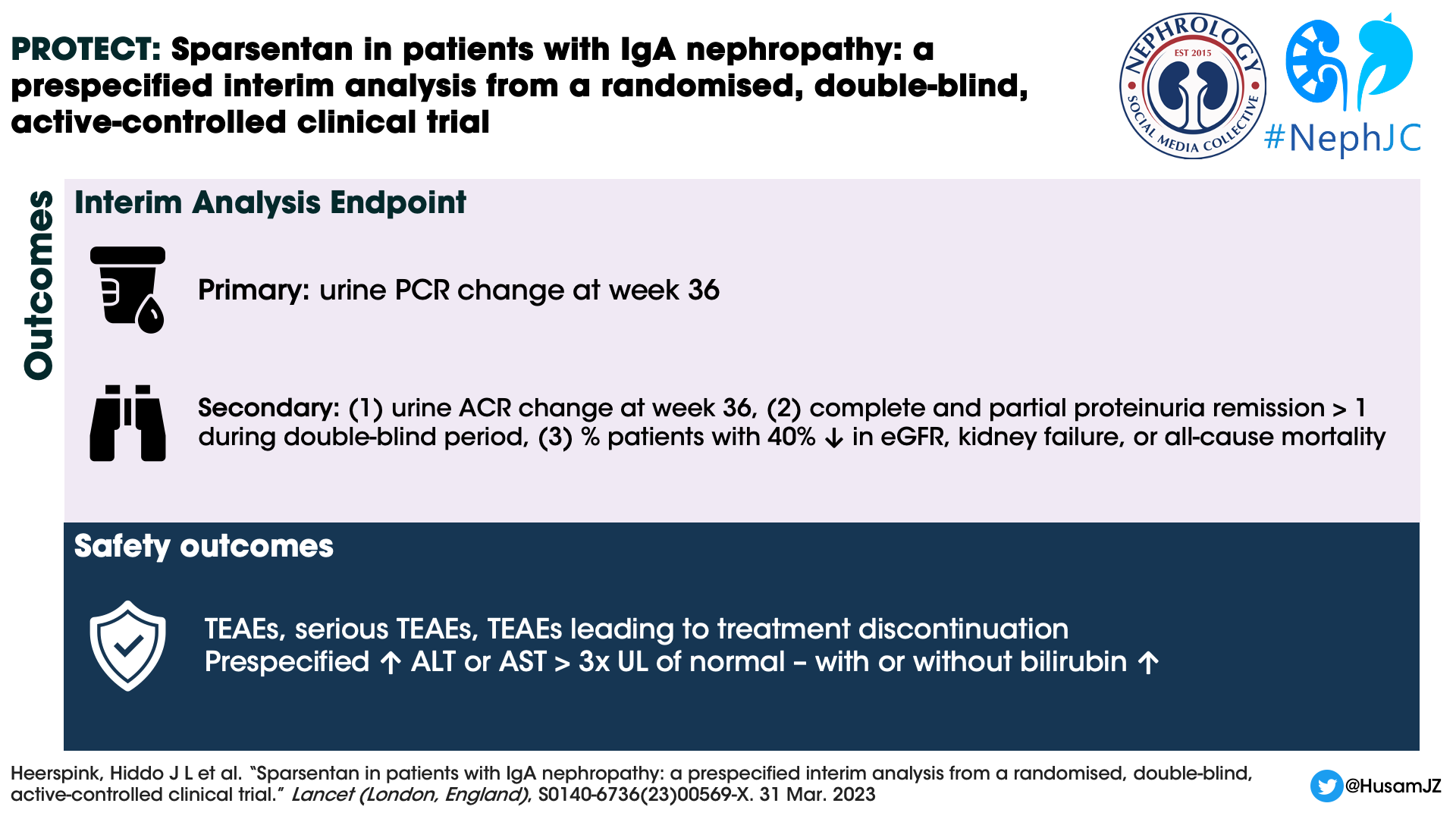
Outcomes
Primary efficacy endpoint:
Change from baseline in urine protein–creatinine ratio (UPCR) based on a 24-h urine sample at week 36
Secondary efficacy endpoints:
Change from baseline in urine albumin–creatinine ratio (UACR) based on a 24-h urine sample at week 36, and
Prespecified exploratory endpoints of complete (UPCR <0.3 g/day) and partial (UPCR <1.0 g/day) remission
Proteinuria remission at least once at any time during the double-blind period
Proportion of patients reaching a confirmed 40% reduction in eGFR from baseline, kidney failure (defined as initiation of kidney replacement therapy or sustained eGFR value of <15 mL/min per 1.73 m2), or all-cause mortality
Safety outcomes
TEAEs were coded with the MedDRA Dictionary (version 23.0)
Treatment Emergent Adverse Events (TEAEs)
Serious TEAEs
TEAEs that led to treatment discontinuation
Prespecified liver function testing, adverse events of interest - ALT or AST > 3 times the ULN with or without elevation of total serum bilirubin > 2 times the ULN
Changes from baseline in bodyweight, SBP and DBP
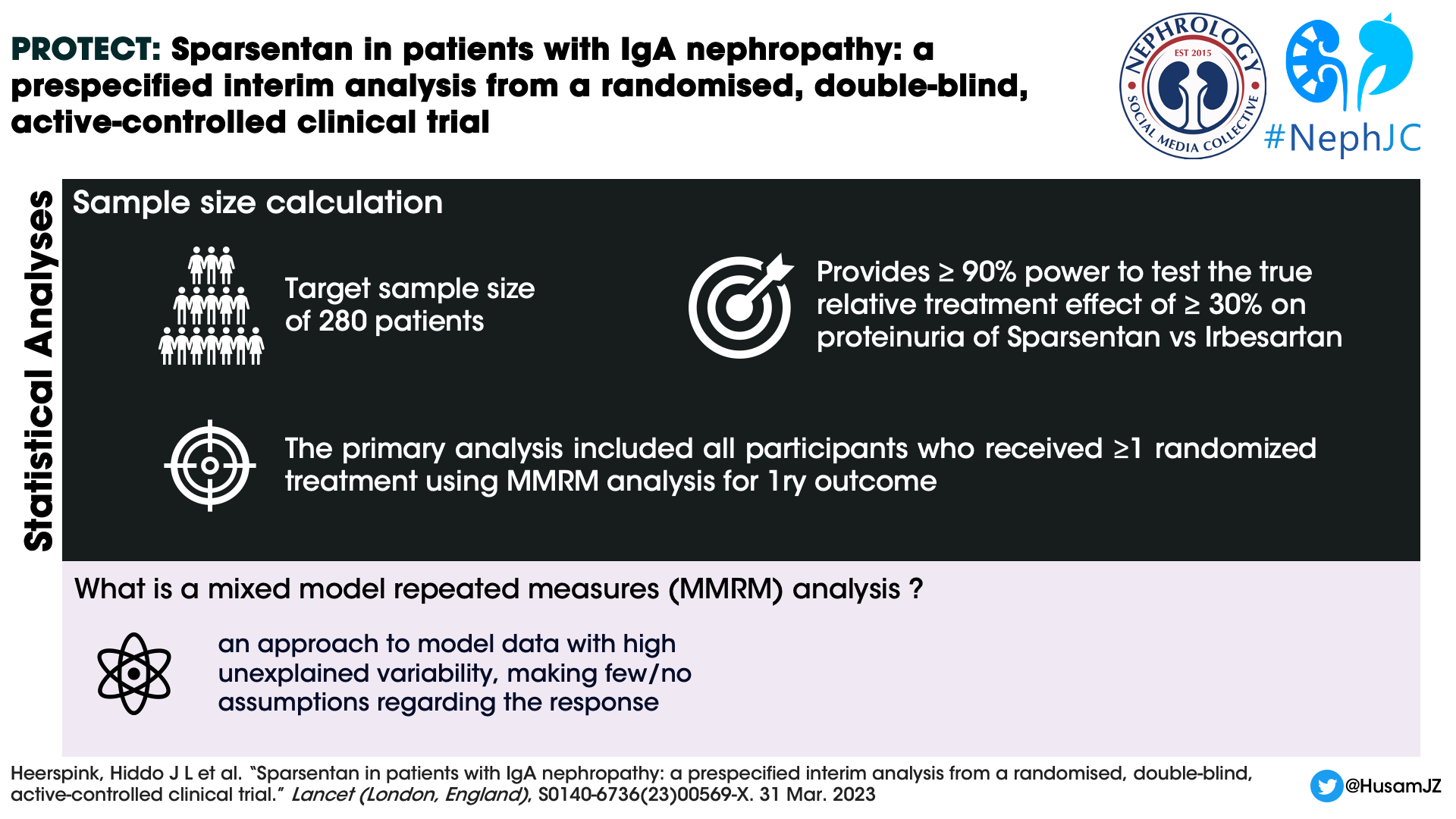
Statistics and Analysis
A sample size of 280 randomly assigned participants was required to test the true relative treatment effect of at least 30% for sparsentan versus irbesartan on proteinuria. For the planned secondary efficacy endpoint of rate of change of eGFR (total eGFR slope) at the completion of the double blind period, a sample size of 380 randomly assigned participants was needed to detect an underlying treatment effect of 2.9 mL/min per 1.73 m2 per year.
The study used mixed model repeated measures (MMRM) analysis to assess changes in UPCR from the beginning of the study to week 36. Available data from all participants were analyzed up to the cut off point. The model considered several factors including the treatment group each participant was assigned to, the baseline UPCR value, time interaction and the randomization stratification. Missing data were attributed using the multiple imputation procedure for the UPCR in the natural log scale. The treatment effect was calculated as the difference between sparsentan and irbesartan as geometric least squares means at week 36. The authors conducted additional sensitivity analysis using the tipping point approach for the robustness of analysis without imputing missing data.
Funding
Travere Therapeutics contributed to study design, data collection, data analysis, and data interpretation. Five authors who were employees of the study sponsor, also participated in writing, review, and approval of the manuscript.
Results
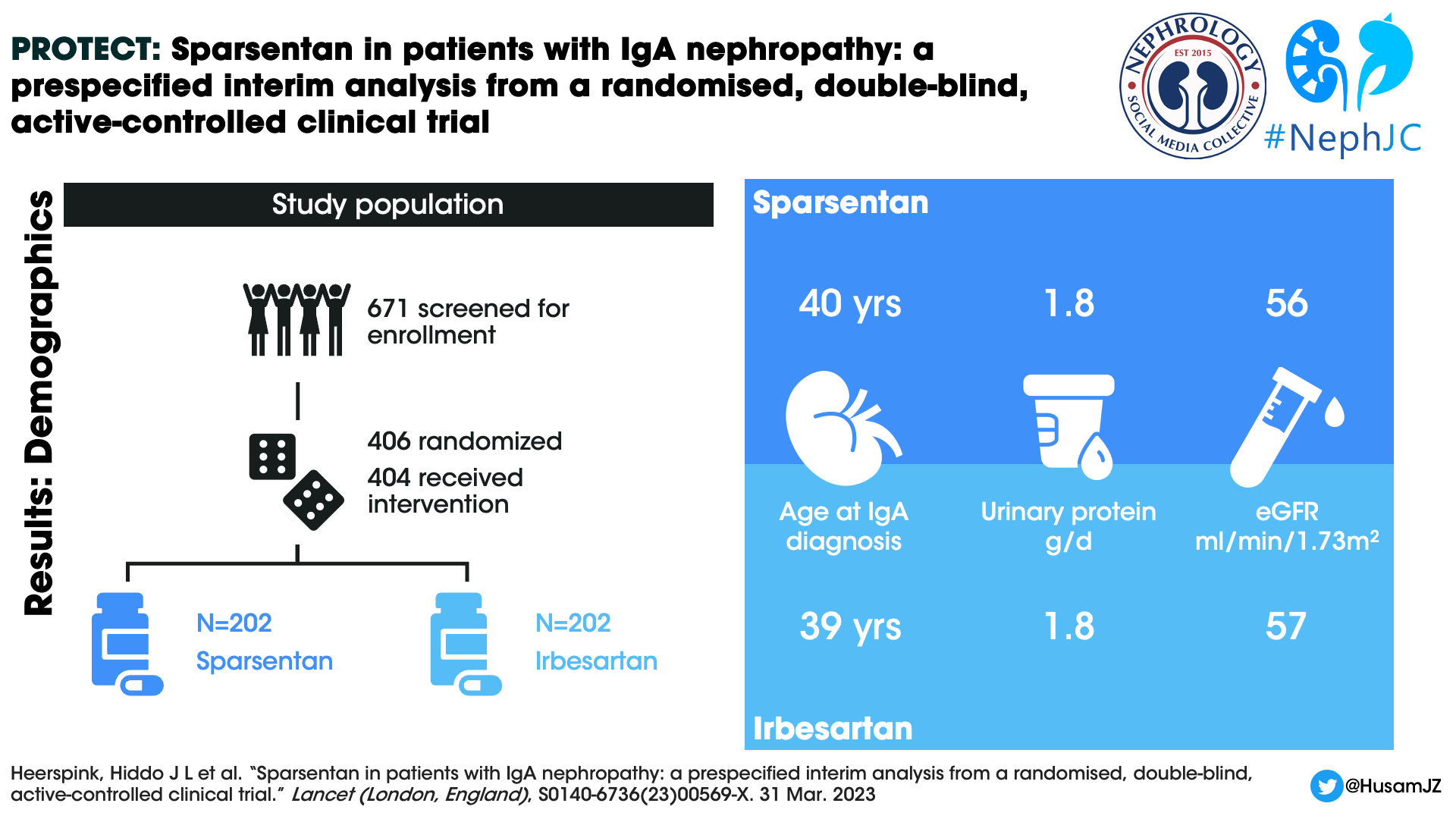
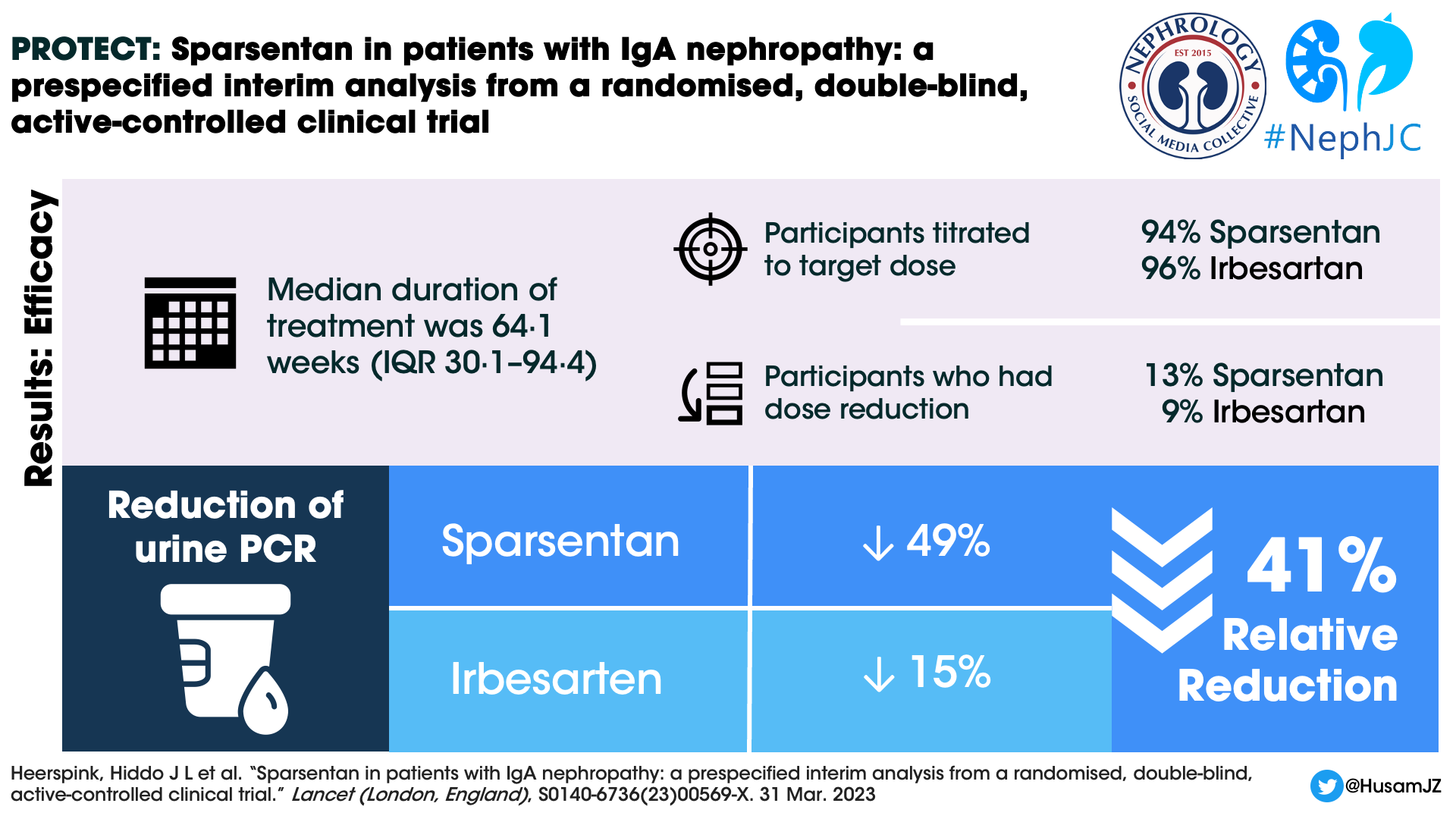

A total of 671 individuals were screened and 404 patients were randomized and received at least 1 dose of the study drug, 202 participants in each arm. The median duration of treatment at the interim efficacy data cutoff was 64.1 weeks (IQR 30.1–94.4). 189 (94%) participants in the sparsentan group and 194 (96%) participants in the irbesartan were titrated to the target dose at the end of two weeks. 26 (13%) and 19 (9%) participants respectively in the sparsentan and irbesartan arms had dose reductions after titration to the target dose.
Figure 1: Trial profile from Heerspink HJL et al, Lancet, 2023
All patients who took at least one dose of randomized therapy were analyzed based on treatment assignment.
Demographics
The mean age of the study participants was 46 years, mean eGFR 57 mL/min/1.73m2, and median UPCR 1.8 g/day. Males constituted 69.8% of the study population. 28.7% were Asians and white population constituted 67.3%. The median time from kidney biopsy to informed consent was 6.4 years.
Table 1: Baseline characteristics from Heerspink HJL et al, Lancet, 2023
96.5% and 99% of the patients were on > 50% maximal labeled dose (MLD) of ACEi or ARB respectively. 24 patients were on both ACEi and ARB.
Figure 1 from Barratt J et al, Kidney Int Rep. 2023
Efficacy
At week 36, the UPCR reduction was 49.8% in the sparsentan group compared to 15.1% in the irbesartan group (Figure 2 below). The Geometric least squares mean ratio between the groups was (sparsentan/irbesartan) 0.59 (CI 0.51-0.69). There was a 41% relative reduction in UPCR in the sparsentan group. The reduction in UPCR was observed in the first post randomization visit at 4 weeks and maintained throughout the follow up period.
During the interim analysis, the final evaluation of the 2-year confirmatory eGFR endpoint was not available. However, a secondary endpoint was assessed, which consisted of a confirmed 40% reduction in eGFR from baseline, kidney failure, or all-cause mortality. A total of 20 patients reached this secondary endpoint with a numerically lesser number of 7 (3%) participants in the sparsentan group. In comparison, the irbesartan group had 13 participants (6%) who reached this endpoint.
Figure 2: Percent change from baseline in urine protein–creatinine ratio in the sparsentan vs irbesartan treatment groups at week 36 and by visit from Heerspink HJL et al, Lancet, 2023
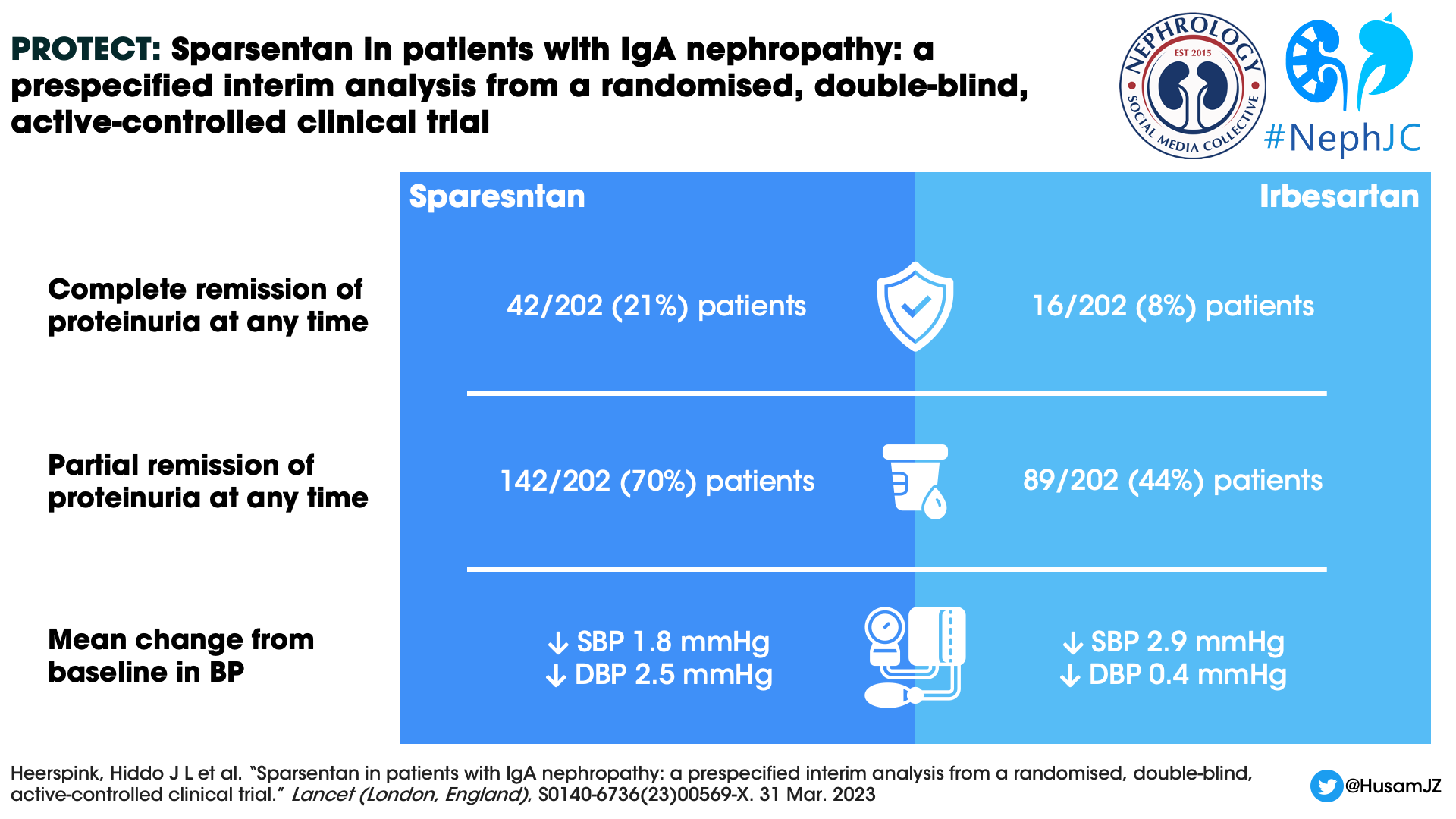
Proteinuria remission
The odds ratio for the complete remission and partial remission of proteinuria at any time over the course of the treatment period was 3.1 (95% CI 1.6–5.8) and 4.5 (95% CI 2.7–7.6) respectively, both in favor of the sparsentan group (Figure 3).
Figure 3: Percentage of patients with complete and partial remission of proteinuria at any time during treatment in the double-blind period from Heerspink HJL et al, Lancet, 2023
Subgroup analysis
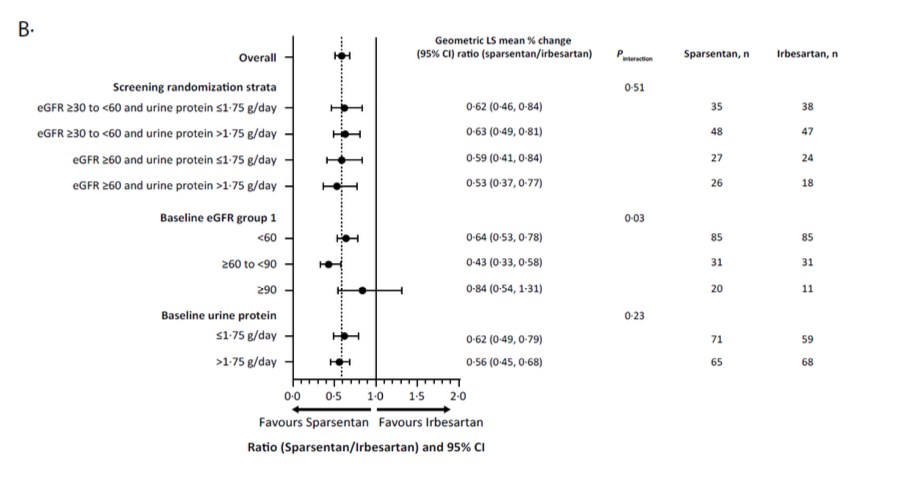
The UPCR benefit was seen across all subgroups (Supplemental Figure 3) defined by baseline demographic, clinical characteristics and concomitant medication use. For eGFR the benefit seems different in the 60-90 subgroup and in the <60 sub group compared to the >90 subgroup.
Supplemental Figure 3: Subgroup analyses from Heerspink HJL et al, Lancet, 2023
Safety
The proportion of patients with any TEAEs (Treatment Emergent Adverse Events) was surprisingly high in both the groups with astronomical figures of 88% in Sparsentan and 78% in the irbesartan group! The TEAEs with ≥ 4% difference included peripheral edema, hypotension including orthostatic hypotension and dizziness. Serious TEAE was seen in 1/5th of all participants and occurred almost similarly in both groups. No deaths were reported in the sparsentan group and one death in the irbesartan group. TEAEs leading to treatment discontinuation occurred in 15 (7%) sparsentan and 10 (5%) Irbesartan group participants. Diuretic initiation was required in 18% and 19% respectively of the participants in the sparsentan and irbesartan groups. Furosemide was most commonly prescribed. Transaminase elevations were similar between groups and there were no cases of hepatotoxicity reported. Also, there was no difference in mean potassium concentrations between the groups.
Table 2 TEAEs reported in ≥ 5% of participants and serious TEAEs reported in two or more participants from Heerspink HJL et al, Lancet, 2023
Next addressing the elephant in the room relating to ETAs- Blood pressure and weight gain! There was no difference in the mean BP between the groups. However, the participants receiving sparsentan showed greater change in DBP from baseline to 36 weeks. The mean change in the body weight from baseline was not statistically significant between the groups
Figure 4. Change in SBP, change in DBP, and blood pressure by visit in the sparsentan and irbesartan treatment groups from Heerspink HJL et al, Lancet, 2023
Discussion
The interim analysis of PROTECT at 36 weeks reveals early (at 4 weeks) and sustained reduction in proteinuria in the sparsentan group as compared to the irbesartan group (↓uPCR -49.8% vs 15.6% respectively, p<0.0001) among patients with IgAN with higher risk of progression (proteinuria > 1g/day despite maximized RASi for 12 weeks). Moreover, the rate of complete (<0.3 g/d) and partial (<1 g/d) remission was significantly higher in the DEARA group. OR for CR =3.1 (p=0.0005) and for PR=4.5 (p<0.0001).
Among trials in IgAN, PROTECT has a fairly large cohort, with 671 patients screened and 404 being randomized. The representation of Asian (29%) and Caucasian (67%) populations was quite broad in PROTECT, similar to DAPA-CKD and in stark contrast to the STOP IgAN, which took place in 32 centers across Germany.
From: Barratt J. et al, Kidney Int Rep (2023)
It is also remarkable that Sparsentan is compared here with maximally tolerated dose of Irbesartan instead of a placebo. The molecular structure of sparsentan has a part of irbesartan! Despite a maximally tolerated RASi for 12 weeks, patients in the Irbesartan group demonstrated a further reduction in uPCR by -15.1%. This probably indicates a gap in the optimization of RASi in the run-in phase. In comparison, the STOP IgAN had >30% patients on dual RASi as compared to 5.9% in the PROTECT trial and around 30% patients were excluded in the run-in phase itself due to remission.
From: Barratt J. et al, Kidney Int Rep (2023)
After the KHI workgroup report on using proteinuria as a “reasonably likely surrogate” end point for kidney preservation, pharma has herded the trials in that direction (Barratt et al, 2019). This is the reason why sparsentan, which has not yet demonstrated a benefit in prolonging renal survival was granted accelerated approval by FDA in high risk IgA (proteinuria >1.5 g/d) while it was denied the same in FSGS (press release says no GFR benefit in DUPLEX study). The KHI report on which this approval is probably based is not without its limitations, one of which is that the minimum duration of proteinuria reduction that confers a protective effect is not known. A small hiccup in this FDA approval is the requirement to check AST/ALT every month for a year and quarterly thereafter. However, with sparsentan this risk was comparable to the control arm in PROTECT, and no patient required discontinuation due to liver injury.
In the DUET study of sparsentan vs irbesartan in FSGS, while sparsentan has demonstrated an overwhelming reduction in proteinuria, the effect is not translated to the better preservation of GFR (Trachtman et al, 2018) Even dual RAS blockade has not demonstrated benefit in eGFR in IgAN (Lennartz et al, 2020). So, whether ETA + ARB would demonstrate benefit, is not known and left unanswered, even after the interim analysis of this study. Whether the FDA would have granted approval to a drug in IgA if it reduced proteinuria but did not decidedly preserve GFR, is a question for another day.
A unique observation here is the reduction in proteinuria without major difference in BP from the control arm (in DUET, BP as well as proteinuria reduced). Though the DBP was slightly lesser in the sparsentan group, mean BP was similar. This points towards a non-hemodynamic antiproteinuric mechanism of the drug in IgAN.
Based on a presentation by Dr. Bruce Hendry
Side-effects known with endothelin receptor antagonists (fluid overload, hypotension and elevation of transaminases) were mostly non-severe and similar to the control group. However, this must be taken with a pinch of salt as both groups had a very high incidence of adverse events (88% in sparsartan & 78% in irbesartan group). There were no fluid overload-related SAEs such as heart failure. Even the use of diuretics was similar (18% in sparsartan vs 19% in irbesartan). The participants were carefully selected to avoid such SAEs, which will have to be kept in mind while applying these results to the broader IgAN population.
Another conflicting situation with the trial is the exclusion of patients on flozins in the initial phase. The improved outcomes in a sizable number of IgAN patients in DAPA-CKD and EMPA-KIDNEY (which came after PROTECT was planned/initiated) now mean they are part of SoC with RASi for IgAN. Will there still be an added benefit of DEARA on this masterly combination of RASi + Flozins? This remains to be seen and is to be addressed in the open label expansion phase where the most recent trial amendment allows for the addition of flozins. Intriguingly, SGLT2is may also possibly mitigate the side-effects of edema caused by DEARA and reduce the use of diuretics (e.g. see ZENITH-CKD trial design).
Patients with crescentic IgA and eGFR <30 have been excluded from most trials as in this one since those patients are considered to need immunosuppression. Timing of kidney biopsy was not a recruitment criterion here, nor was the degree of histopathological severity, except that crescentic IgA has been completely excluded. Microscopic hematuria, which denotes ongoing inflammation, could not be tested here because the investigators used a central lab for urine examination and transport of the sample would damage it.
A few ongoing trials with sparsentan are shown in this figure:
Overall, sparsentan has demonstrated reasonably good efficacy in reducing proteinuria and can be presented as an option to pure RASi. Whether it can be added to existing RASi is unanswered, however the risk (AKI, hyperkalemia) will have to be weighed against the benefit. Also, if and when to switch from RASi to DEARA will have to be judged. Most importantly, it seems this DEARA will be priced quite dearly ( $9900 per month) which seems somewhat steep for proteinuria reduction, while the GFR data is still awaited.
CONCLUSION
Sparsentan (ET1 + AT1 blocker) when compared to Irbesartan (ARB) demonstrated:
1. Favorable effects on proteinuria reduction (49.8% vs. 15.1%) among patients with IgAN with proteinuria >1g & eGFR >30 ml/min/1.73 m2
2. Comparable side effect profile
3. ESKD or 40% decline in GFR – uncertain as of now
Summary prepared by Anitha Swamy,
Assistant Professor,
All India Institute of Medical Sciences,
Hyderabad, India
And
Saumya Vishnoi
Clinical Associate,
Institute of Renal Sciences, Global Hospital,
Mumbai, India
NSMC Interns, Class of 2023
Reviewed by Cristina Popa, Sandhya Suresh,
Sayali Thakare and Swapnil Hiremath
Header Image created by AI, based on prompts by Evan Zeitler