#NephJC Chat
Tuesday, July 8th, 2025, 9 pm Eastern on Bluesky
N Engl J Med. 2025 Jun 5. doi: 10.1056/NEJMoa2410659. Online ahead of print.
Finerenone with Empagliflozin in Chronic Kidney Disease and Type 2 Diabetes
Rajiv Agarwal, Jennifer B Green, Hiddo J L Heerspink, Johannes F E Mann, Janet B McGill, Amy K Mottl, Julio Rosenstock, Peter Rossing, Muthiah Vaduganathan, Meike Brinker, Robert Edfors, Na Li, Markus F Scheerer, Charlie Scott, Masaomi Nangaku; CONFIDENCE Investigators
PMID: 40470996
Introduction
Prescribing patterns have changed significantly for diabetic kidney disease (DKD) in the last decade. No longer are we constrained solely to the use of renin-angiotensin inhibitors (RASi) as our only hope for slowing the decline in eGFR. The care of patients with DKD now rests on 4 well-defined pillars - RASi, sodium-glucose cotransporter-2 inhibition (SGLT2i flozination), non-steroidal mineralocorticoid antagonism (nsMRA, namely finerenone), and glucagon-like peptide 1 receptor agonists (GLP1-RA). The direct confirmation of the nephroprotective effects of these medications comes from dedicated kidney outcomes trials such as FLOW (NephJC Summary), FIDELIO-DKD (NephJC Summary), FIGARO-DKD, CREDENCE (NephJC Summary), DAPA-CKD (NephJC Summary), and EMPA KIDNEY (NephJC Summary); you can tell we have been very excited by these advances by the number of NephJCs done on these classes of medications!
Although SGLT2i/GLP1-RAs were not the standard of care at the time of conception of the finerenone trials, the FIDELITY analysis (a pooled analysis of the FIGARO-DKD and FIDELIO-DKD trials) deserves more than just a retrospective glance. It was a reassuring signal, as finerenone improved kidney and cardiovascular outcomes regardless of background SGLT2i use, hinting at additive effects between finerenone and flozins on kidney and CV outcomes in type 2 diabetes mellitus (T2DM).
Slide from Johannes Mann’s presentation, during CONFIDENCE presentation, Late Breaking Clinical Trials 1, 62nd European Renal Association Congress
The signal of additive benefit, however, was not new. A small trial, ROTATE-3, had already shown that combining a flozin with the MRA eplerenone produced a meaningful, additive reduction in albuminuria (over 50% in just 4 weeks), compared to either agent alone (Provenzano M, et al, JASN, 2022).
However, the question remains: should we sequentially add these medications to combat DKD, or could we become more like the HFrEF doctors and initiate them simultaneously? A concurrent start of some pillars would help overcome “therapeutic inertia” and fragmented clinic visits. It’s time to discuss how much CONFIDENCE we have in initiating SGLT2-i and nsMRAs simultaneously because after all, time is kidney…
The Study
Design
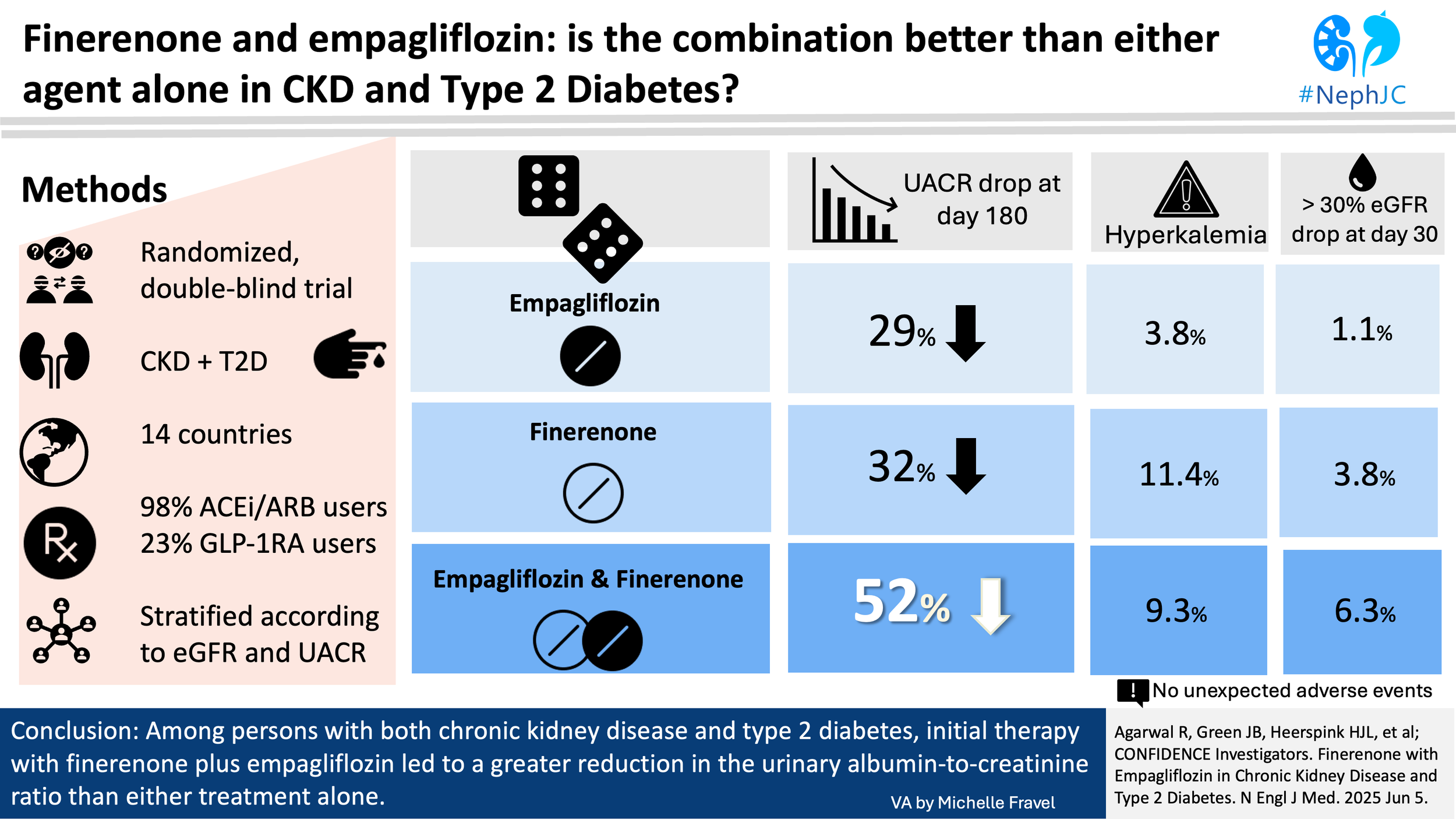
This was a parallel-group treatment, double-blind, international, randomized trial. It was conducted to assess efficacy in reducing urinary albumin to creatinine ratio (UACR) from baseline, and safety of finerenone plus empagliflozin compared with either finerenone or empagliflozin alone in patients with chronic kidney disease (CKD) and T2DM.
Study population
Inclusion criteria:
Adult
CKD with an eGFR 30-90 ml/min/1.73m^2 (using Scr and CKD- EPI 2009 Equation) and at least one historical value of eGFR <60 mL/min/1.73 m^2
UACR 300-5000 mg/g (34- 565 mg/mmol) within 3 months prior
T2DM with A1c <11% (97 mmol/mol)
Receiving stable (1 month prior to screening), max tolerated dose of either ACEi or ARB, but not both
Exclusion criteria:
Type 1 Diabetes Mellitus
Cardiovascular: HFrEF (NYHA class II - IV), recent CV events (stroke, MI, or HHF within 90 days)
Patients with a serum potassium above 4.8 mmol/L at screening
Renal: significant renal artery stenosis (>75%), non-diabetic kidney disease, recent AKI (within 6 months), prior kidney transplant.
Concomitant medications: recent SGLT2i (within 8 weeks prior), MRAs, potassium supplements, potassium binders, or ARNI (within 8 weeks), strong CYP3A4 inhibitors or inducers (within 7 days)
Other exclusions: advanced liver disease (Child Pugh Class C), BP reading at day 1 visit higher than 160/100 or SBP <90, recent major surgery, pregnant and/or breastfeeding
Intervention
Eligible patients were randomized (1:1:1) to either receive finerenone (10 mg or 20 mg), empagliflozin (10 mg), or finerenone and empagliflozin combined. The starting dose of finerenone was 10 mg if eGFR less than 60 or 20 mg for eGFR ≥ 60 mL/min/1.73 m². Participants receiving finerenone 10 mg could be uptitrated to 20 mg anytime after day 30 if the potassium was ≤ 4.8 mmol/L and the eGFR decline from the prior trial visit was < 30%.
The randomization was stratified by eGFR (<60 or ≥60 mL/min/1.73 m²) and UACR (≤ 850 mg/g or > 850 mg/g).
Figure 1-1 (from Protocol). Overall study design, Agarwal R, et al. N Engl J Med, 2025
Primary Efficacy Outcome
The relative change from baseline in UACR at 180 days in the combination therapy group vs either empagliflozin alone or finerenone alone.
This was a practical, short-term surrogate marker requiring only 807 participants. Hard outcome alternatives could have been a kidney composite outcome, including kidney failure, eGFR decline ≥ 40%, or kidney-related death. It would need approximately 41,000 participants and 5+ years of follow-up to achieve statistical power for these definitive clinical endpoints, considering the active comparators (i.e. no double placebo arm).
Adapted from table 4, Green JB et al, Nephrol Dial Transplant, 2023
Secondary Efficacy and Safety Outcomes
Efficacy: proportion achieving UACR reductions ( >30%, >40%, >50%)
Safety: AKI, hyperkalemia, change from baseline in the serum potassium level, symptomatic hypotension, ketoacidosis, severe hypoglycemia, and genital mycotic infections
Post-treatment effects: UACR and eGFR changes after drug discontinuation (day 180 to 210).
Sample size and analytic plan
Although for primary outcome, the primary analysis used a mixed-model for repeated measures (MMRM), the sample size was conservatively calculated using a two-sample t-test, assuming equal variance and a log-normal distribution of UACR.
Finerenone and empagliflozin were expected to reduce UACR to 62.8% (based on FIDELIO- DKD at 6 months) and 70% (based on EMPA-REG) of baseline, respectively, with the combination hypothesized to achieve an additional 20% reduction, yielding UACR ratios of approx. 0.50, and 0.56. Using an SD of 0.77, the study was powered at 80% to detect log-transformed mean differences of -0.224 and -0.223, respectively.
Adjusting for multiplicity (Holm-Bonferroni; alpha = 0.025 per comparison), the planned sample size was 228 participants per group (684 total), with an inflated enrollment of 807 (260 per group) to account for a 15% dropout rate.
Population: Three predefined analysis sets were used. The full analysis set (FAS) included all randomized patients, according to the intention-to-treat principle. The safety analysis set included all individuals who received at least one dose of study medication. The per-protocol set included participants from FAS who adhered to the treatment regimen without major protocol deviations.
Primary endpoint: The primary analysis used a repeated measure model that adjusted for the treatment group, study visits, and their interaction, as well as baseline UACR and the values used to stratify randomization (eGFR and UACR categories). The treatment effect was expressed as a least-squares mean ratio, derived from MMRM analysis, representing the adjusted proportional change in UACR between groups over time. Missing data were handled using multiple imputation, assuming data were missing at random. Additional sensitivity analyses explored less favorable assumptions (eg, data not missing at random) and looked at outcomes limited to the treatment period and the 30 days following discontinuation. The main comparison was combination therapy vs each monotherapy; the two monotherapies were not directly compared.
Secondary analyses: Logistic regression models were used for categorical response analyses. For safety outcomes, event rates were analyzed descriptively and compared using chi-square or Fisher’s exact test.
Sensitivity analyses confirmed robust results using different missing data methods and the updated CKD-EPI GFR equations. Subgroup analyses were done for regions, kidney function, cardiovascular history, blood pressure, potassium, age, and gender.
Protocol deviations: participants with major protocol deviations were excluded from the per-protocol analysis. This included cases involving Good Clinical Practice violations. Specifically, data from site 20003 in Japan were excluded due to noncompliance with trial conduct standard.
Funding
The sponsor, Bayer (manufacturer of finerenone), provided financial support for editorial and medical writing assistance and participated in the decision to submit the manuscript. The initial draft was written by the lead author, who also independently analyzed and verified the data. All authors affirmed the accuracy and completeness of the data, and both the sponsor and investigators confirmed adherence to the study protocol.
Among the 15 authors, 5 were employees of Bayer. An additional 5 authors served as consultants or steering committee members for Bayer in connection with the trial. 9 authors disclosed financial relationships with Boehringer Ingelheim (co-developer of empagliflozin), including roles such as consultant, grant recipient, or advisory board member. 6 authors reported financial ties to Eli Lilly, also involved in the development of empagliflozin; these included consulting relationships, research funding to institutions, or company stock ownership.
Results
A total of 800 participants (48%) out of 1664 screened were randomized and included in the full analysis population for efficacy assessments, indicating that nearly half of those screened met the eligibility criteria.
269 in the combination therapy group
264 in the finerenone group
267 in the empagliflozin group
746 participants completed the full study including the 30-day follow-up period.
Figure S2. CONSORT diagram. Agarwal R, et al. N Engl J Med, 2025.
The mean age was 68 years, the mean BMI was 29.8 ± 6.7, and the eGFR averaged 54 ± 17 mL/min/1.73 m², with about 65% of the patients having CKD stage 3. Median UACR was 574 mg/g (IQR 274–999), equivalent to 65 mg/mmol (IQR 31–113); median serum potassium was 4.4 ± 0.4 mmol/L. The mean HbA1C was 7.3 ± 1.2% (56 ± 13 mmol/mol), and 28% of patients had atherosclerotic cardiovascular disease. RASi use was near-universal at 99.3%. In addition, statins were used by 78%, GLP-1RA in 25%.
Table 1. Demographic and clinical characteristics. Agarwal R, et al. N Engl J Med, 2025.
Primary outcome
At day 180, combination therapy led to a 29% greater reduction in UACR compared to finerenone alone (least-squares mean ratio 0.71; 95% CI, 0.61-0.82;) and a 32% greater reduction compared to empagliflozin alone (least-squares mean ratio 0.68; 95% CI, 0.59–0.79). These findings suggest an additive effect with the combination approach. After stopping treatment at day 180, UACR levels rebounded by day 210 across all groups.
Figure 1A. Least-squares mean ratio of the change from baseline in the urinary ACR in the full analysis population Agarwal R, et al. N Engl J Med, 2025.
Figure 1B. Mean serum potassium level at each visit in the safety analysis population Agarwal R, et al. N Engl J Med, 2025.
Adverse events were reported in over half of participants across all groups, with serious adverse events occurring in 7%. Treatment discontinuation due to adverse events was slightly more frequent with combination therapy (4.5%) compared to monotherapies (3.4%). Hyperkalemia was more common with finerenone (11.4%) and combination therapy (9.3%) than with empagliflozin (3.8%). Serum potassium >5.5 mmol/L occurred in 18.6% (finerenone), 15.3% (combination), and 9.7% (empagliflozin); potassium >6.0 mmol/L was seen in 4.6%, 4.6%, and 2.7%, respectively.
Table 3. Adverse Events and Safety Assessments after Treatment Initiation Agarwal R, et al. N Engl J Med, 2025.
A greater than 30% decline in eGFR at 30 days was noted in 6.3% with combination therapy, compared to 3.8% and 1.1% with finerenone and empagliflozin, respectively.
Figure 1C and 1D. Mean serum potassium level at each visit in the safety analysis population Agarwal R, et al. N Engl J Med, 2025.
An early decline in eGFR was observed across all groups, which was largely reversible after treatment discontinuation. Systolic blood pressure decreased most with combination therapy (–7.4 mmHg by day 30), then rose back to baseline after drug discontinuation. Finerenone and empagliflozin monotherapies had smaller effects on blood pressure. No significant differences in HbA1c were observed between groups at 180 days.
Figure S4. Changes in HbA1C at each visit in the safety analysis population Agarwal R, et al. N Engl J Med, 2025.
Genital mycotic infections occurred in only 1.5% of participants in each of the empagliflozin-containing groups - a notably low rate given the background of diabetes in all participants. Symptomatic hypotension was reported in just 1% of participants in the dual-therapy group. Importantly, no new-onset heart failure events were observed in over 700 participants with diabetes and CKD during the 6-month study period.
Discussion
It seems like we can have some CONFIDENCE in upfront combination therapy. There was a significant benefit in terms of the primary outcome: at day 180, the reduction of UACR with the combination therapy was 29% greater than that of finerenone alone and 32% greater than that of empagliflozin alone. So similar to GDMT in HFrEF, seen in trials such as STRONG-HF and PIONEER-HF, more aggressive dual initiation can be viable within nephrology too. This provides us a roadmap to begin nephroprotective agents earlier and in combination to decrease the risk of “therapeutic inertia.”
However, most of us won’t be really surprised by the efficacy results - as mentioned in the introduction, we’ve had several sources of data suggesting synergy on UACR reduction with MRAs and flozins. Not only that, but we have the hard outcome data which shows that patients do better when on multiple pillars (Neuen, et al, Circulation, 2024). So as important as the positive outcome on uACR in CONFIDENCE was the safety data - really, this was a safety trial to tell us what to expect should we employ dual medication starts in clinical practice.
What about combo safety?
While all groups faced an early dip in eGFR, there was a larger decline in eGFR seen with combination therapy. Reassuringly on average this stabilized around day 30, and was reversible with drug discontinuation at 180 days.
However, it is important to note there was a decrease in eGFR >30% at day 30 in 17 out of 268 participants (6.3%) in the combination group compared to the 3.8% in the finerenone group and 1.1% of the empagliflozin group. To put this in context, from the mean starting eGFR of 54 ml/min/1.73 m², you would expect the initial drop in 6.3% of patients to put them to eGFR <38 ml/min/1.73 m². It is unclear if these patients permanently discontinued trial treatment or if they simply continued treatment and this initial decrease stabilized - in a trial as much about safety as efficacy, it would be helpful to have more information about this group of patients.
Patients had a systolic blood pressure (SBP) baseline of 135 mm Hg. The greatest reduction in blood pressure was in the combination therapy group (-7.4 mm Hg), which was greater than each monotherapy, but still the rate of symptomatic hypotension was very low at 1%, which was reassuring to see.
As we would expect, the patients on finerenone experienced more hyperkalemia, similar to what we saw in previous trials. The mean increase of potassium was 0.27 mmol/L after 14 days of initiation of combination therapy, which did decline to near baseline 30 days after treatment was stopped. Flozins are known to modestly decrease hyperkalemia rates (Neuen et. al, 2024). In this trial, there were fewer investigator-reported hyperkalemia when empagliflozin was combined with finerenone (see slide below), but when you look at Table 3 above, unfortunately, there were no fewer episodes of potassium >6 mmol/L in the combination group in this trial, which would have been a bonus win for flozination if it had been seen.
Slide from Rajiv Agarwal’s presentation, during CONFIDENCE presentation, Late Breaking Clinical Trials 1, 62nd European Renal Association Congress
Strengths and limitations
Almost all (98.4%) of participants were on a maximally tolerated ACE-i or ARB, as patients with DKD should ideally be
The patient population also included a higher percentage of Asian participants (46%) compared to other trials such as DAPA-CKD and EMPA- KIDNEY (~10-20%). Asians are prone to higher rates of diabetes-related complications. While often lean by BMI, they accumulate visceral fat which promotes insulin resistance and metabolic harm. This results in diabetes often going undetected until damage begins- hence screening guidelines suggesting DM screening at BMI ≥23 kg/m², not 25 kg/m². With about 60% of the world’s population living in Asia and the increasing burden of diabetes it is important to assess safety and efficacy in these populations. (Guarigata L, et al, 2013)
However, the trial recruited less than 25% of females. In 2025 this is woeful, and investigators need to step up to improve this consistent under-recruitment of females across cardio-metabolic-renal trails.
This will surely have to be the final trial still recruiting patients with 2025 that not only omits flozins in patients with DKD in the name of comparison but actually stops them for a month at the end of the trial to record what happens.
Association is not causation, but…
To start, UACR served as a good biomarker in this trial, we were able to see the sustained decrease of combination therapy through day 180. There was also adequate time to see the initial dip in eGFR stabilized around day 30. While groups were not followed long enough to evaluate for differences in cardiovascular outcomes or kidney disease progression, it is not that extending this trial would be beneficial. Extending a trial like this would result in DKD patients remaining on/off monotherapy despite knowing that they would have better renal preservation on them.
If you’re looking more into rational, sophisticated why, in this case, even if the association is not causation, the finerenone might be the mediator between surrogate and hard endpoints. Using causal mediation analysis, the effect of finerenone on kidney and cardiovascular outcomes was partitioned into
Direct effects: independent of UACR
Indirect: mediated by early change in log UACR (baseline to month 4)
Figure 1. Mediation model, from Agarwal R, et al, Ann Intern Med, 2023
According to a mediation analysis including FIDELIO and FIGARO trials, finerenone increased mean survival time by 15%, of which 84% (CI 48.8-100%) was mediated via UACR reduction. Moreover, a ≥30% UACR reduction explained 63.6% of the kidney benefit and 26.1% of the CV benefit. Therefore, we can easily say that UACR is more than a surrogate.
What does the future withhold?
“The future belongs to those who believe in the beauty of their dreams”
The success of rapid GDMT escalation in heart failure, where four pillars are initiated within weeks, sets the precedent for CKD. CONFIDENCE proves that delaying nephroprotection is riskier than dual therapy, paving the way for a CKD polypill: SGLT2i, finerenone, RAS blockade, and GLP1 RAs combined early, not sequentially. Just as cardiologists abandoned drug cautious uptitration, CKD management must embrace aggressive, simultaneous intervention- because renal decline, like cardiac remodeling, rarely waits for slow drug adjustment. The era of therapeutic inertia should end; the future is maximal, upfront nephroprotection.
Conclusion
The four pillars for treating diabetic kidney disease are here - the exciting thing about this trial is that it informs us how we may be able to improve our implementation of them by looking at how efficacious and safe combining agents in finerenone and empagliflozin can be. They conclude that in patients already on RAS blockade, adding both finerenone and empagliflozin leads to greater proteinuria reduction than either alone, though at the expected cost of increased adverse effects like reversible eGFR decline.
Summary prepared by:
Sejal Lakhani
Internal Medicine Resident,
Lehigh Valley Health Network
Allentown, PA, USA
Jeyakumar Meyyappan
Assistant Professor, Nephrology
SGPGIMS, Lucknow, India
NephJC interns, 2025