#NephJC Chat
Tuesday April, 11th, 2023 at 9 pm Eastern
Wednesday April 12th, 2023, at 9 pm Indian Standard Time and 3:30 pm GMT
N Engl J Med 2023 Mar 2;388(9):781-791. doi: 10.1056/NEJMoa2209275
Hydrochlorothiazide and Prevention of Kidney-Stone Recurrence
Nasser A Dhayat, Olivier Bonny, Beat Roth, Andreas Christe, Alexander Ritter, Nilufar Mohebbi, Nicolas Faller, Lisa Pellegrini, Giulia Bedino, Reto M Venzin, Philipp Grosse, Carina Hüsler, Irene Koneth, Christian Bucher, Rosaria Del Giorno, Luca Gabutti, Michael Mayr, Urs Odermatt, Florian Buchkremer, Thomas Ernandez, Catherine Stoermann-Chopard, Daniel Teta, Bruno Vogt, Marie Roumet, Luca Tamò, Grazia M Cereghetti, Sven Trelle, Daniel G Fuster
PMID: 36856614
Kidney stones are a major problem worldwide which are increasing in prevalence. This week we look at the NOSTONE trial.
Introduction
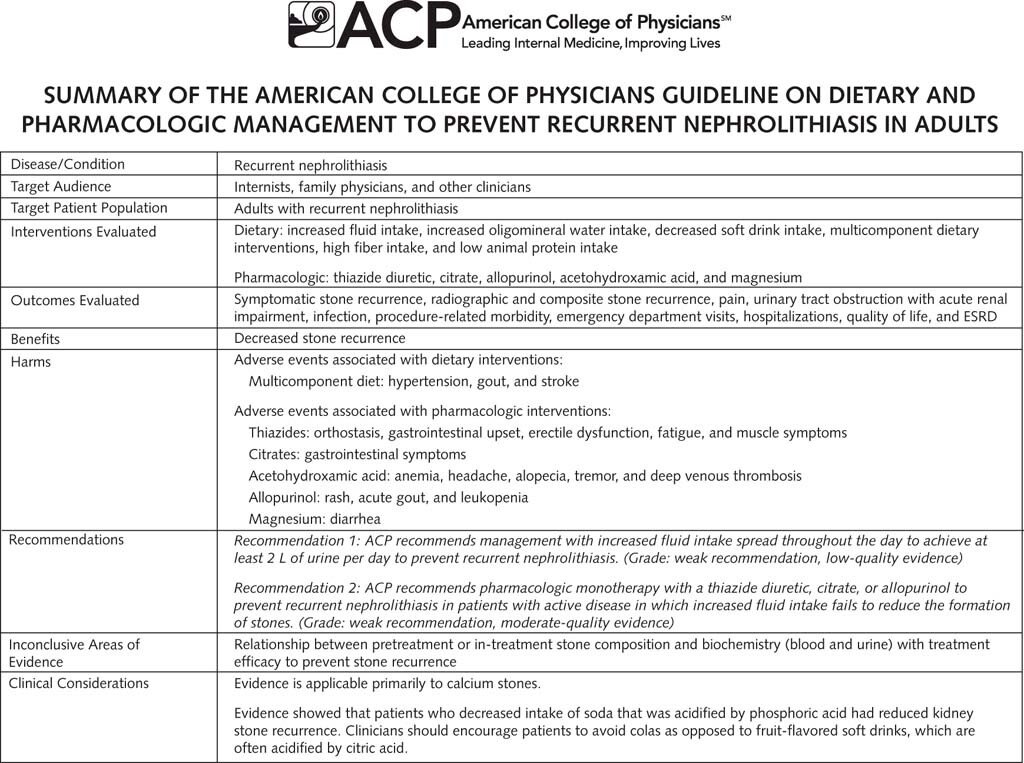
The incidence and prevalence of kidney stone disease are increasing globally, irrespective of age, sex, and race. Without a specific treatment, the 5- and 20-year recurrence rates are ~ 40% and ~ 75%, respectively (Dhayat et al, BMC Nephrol 2018). Prevention of kidney stone recurrence includes behavioral and nutritional interventions as well as pharmacological treatment specific for the patient’s stone. Interventions focus on minimizing urinary supersaturation. More than 90% of kidney stones are formed of calcium oxalate, calcium phosphate or a mixture of both. Hypercalciuria is associated with dietary, familial, and idiopathic sources. However, restriction of dietary calcium is not recommended for stone prevention as it enhances negative calcium balance in the body with increased tendency to form calcium oxalate stones (Taylor & Curhan, J Urol 2013).
Thiazides are time tested drugs (over 50 years) which have shown benefit in preventing stone formation, thought to be due to their action on reducing urinary calcium excretion (Dhayat et al, BMC Nephrol, 2018, see table). The mechanism of lowering urinary calcium, including lowering calcium excretion and increasing calcium reabsorption, can be seen in the figure below.
Schematic showing mechanism of HCTZ action in reducing urinary calcium
Table on previous studies of thiazides in kidney stones (from Dhayat et al et al., BMC Neph 2018)
Although thiazides are a cornerstone of pharmacologic prevention, there is a paucity of data on the efficacy of these agents compared with placebo, as well as limited dose-response data. A meta-analysis (Li et al., J Transl Med 2020) assessing the use of thiazide diuretics for the prevention of recurrent kidney calculi concluded that the quality of evidence is low. The trials done so far are small, often not blinded, and have other methodological weaknesses. Hence the need for a large well designed RCT which would validate a standard, guideline recommended therapy worldwide.
The Study
Methods
NOSTONE is a double blind, randomized, placebo-controlled trial using low to high doses of thiazides to assess their ability to prevent recurrence of kidney stones done in multiple centers in Switzerland.
Study Participants:
Informed consent
Age 18 years or older
Recurrent kidney stone disease (≥ 2 stone events within the 10 years prior to randomization)
Any past kidney stone containing 50% or more of calcium oxalate, calcium phosphate or a mixture of both
Major Exclusions:
Pharmacologic prevention for stone recurrence less than 3 months prior to randomization
Patients with secondary causes of recurrent calcareous nephrolithiasis and those who were on drugs that could interfere with kidney stone formation.
Participants were randomized into 4 groups:
12.5 mg hydrochlorothiazide daily
25 mg hydrochlorothiazide daily
50 mg hydrochlorothiazide daily
Placebo daily
Participants underwent non-contrast CT scan of kidneys at start of randomization and at the end of 3 years. Follow up visits were at 3 months and then yearly. There were telephone visits every 3 months.
Outcomes:
Primary outcome
A composite of symptomatic or radiologic recurrence of kidney stones.
Symptomatic recurrence
The visible passage of a stone with or without accompanying typical symptoms (such as flank or loin pain and hematuria) or a stone which requires surgical intervention.
Radiological recurrence
Appearance of new stones on CT or the enlarged pre-existing stones that had been observed on the baseline CT; details are provided in the protocol.
Secondary outcomes
Individual end points of symptomatic recurrence and radiologic recurrence,
Urine relative supersaturation ratios, which indicate the degree by which the concentration of calcium oxalate or calcium phosphate in urine exceeds the equilibrium solubility, were calculated with the use of EQUIL2 software.
Sample size and analytic plan
The null hypothesis was that there is no association between stone recurrence with HCTZ dose. For this they needed 104 in each group (3 HCTZ doses and a placebo group), to get 80% power. They assumed a 10% drop-out rate, 20% stone recurrence at 1-year and 45% at 3-years, and a relative risk reduction of 10%, 35% and 50% for the HCTZ escalating doses of 12.5, 25 and 50 mg respectively. It was an intention to treat analysis. Stratification was done according to the number of kidney stones episodes in the 10 years prior to randomization. Per protocol analysis was used for the primary outcome. Log rank test was used to analyze for dose response. Recurrence (symptomatic and radiological) was analyzed via logistic regression. Laboratory tests were analyzed by a mixed effect model.
Funding source
This was an investigator initiated trial, funded by the Swiss National Science Foundation and Inselspital.
Results
As planned, 416 patients were enrolled from the 1335 screened. Details of reasons for not being eligible are not provided in the CONSORT diagram (figure S1, below).
The baseline demographics of the study participants are outlined in Table 1. The median age was 49 years (IQR 39-55); 85 patients (20%) were women. The median number of kidney stone events in the 10 years before randomization was 3 (2-4), and ≥4 in 139 patients (33%). At baseline, 258 patients (63%) had hypercalciuria (urinary calcium excretion rate >200 mg/24 hours).
Table 1 from Dhayat et al, NEJM 2023
Nonadherence, defined as missing >20% of doses, was 26% (27 patients, of whom 12 did not adhere for nonmedical reasons) in the placebo group, 15% (16, 3 nonmedical reasons) in the 12.5 mg HCTZ group, 24% (26, 13 nonmedical reasons) in the 25 mg HCTZ group, and 26% (26, 10 nonmedical reasons) in the 50 mg HCTZ group.
Patients on HCTZ had slightly lower urinary calcium excretion vs placebo, but the urine relative supersaturation ratios for calcium oxalate and calcium phosphate were not consistently lower than in the placebo group as can be seen from Table 2. This may be from an increase in oxalate and a decrease in citrate excretion which also contribute to calcium oxalate supersaturation.
Table 2 from Dhayat et al, NEJM 2023
Outcomes
The primary end-point event of symptomatic or radiologic recurrence of kidney stones was not statistically significant (figure 1A). Similarly, for the individual outcomes of symptomatic stone recurrence and radiologic recurrence, there was no statistically significant difference.
Figure 1A, 1B from Dhayat et al, NEJM 2023
The radiological recurrence was lower at 25 and 50 mg doses (figure 1C and Table S12, below), however the trend was not significant for a dose-effect relationship.
Figure 1C from Dhayat et al, NEJM 2023
There was no evidence of a relation between HCTZ dose and the occurrence of a primary end-point event in the intention-to-treat analysis (rate ratio for trend 0.98; 95% CI, 0.87-1.09) or per-protocol analysis (rate ratio 0.98; 0.88-1.09). The results were similar after sensitivity analyses, including restricting to patients without kidney stones at baseline and excluding symptomatic events within the first 6 or 12 months after randomization to allow for washout of preexisting stones.
Subgroup analysis
There was no benefit seen with thiazides in any of the several subgroups examined, as can be seen below.
Figure 2 from Dhayat et al, NEJM 2023
Safety/Adverse effects
New-onset diabetes mellitus, hypokalemia, gout, skin allergy, and creatinine >150% baseline were more common among patients in HCTZ groups than placebo. Note that hyponatremia was not a reported adverse event. The incidence of serious adverse events was not higher among patients on HCTZ than placebo.
Table 3 from Dhayat et al, NEJM 2023
Discussion
The NOSTONE trial reported that treatment with HCTZ did not differ substantially from placebo in preventing symptomatic or radiologic recurrence of kidney stones in patients at high risk for recurrence. Symptomatic recurrence was similar across all groups, and results were confirmed by several sensitivity analyses. However, the incidence of radiologic recurrence, a composite of stone growth or new stone formation, was lowest among patients on 25 mg or 50 mg of HCTZ
Strengths of the trial include being a well conducted and designed trial, being double blind with meticulously arbitrated endpoints, being adequately powered; the enrollment target of 416 patients was met. The result was coherent when one also incorporates the mechanistic analyses of supersaturation.
Limitations include underrepresentation of women and non-white race as most patients were white men. The trial excluded patients with CKD, secondary causes of nephrolithiasis, and those on drugs that could interfere with kidney stone formation.
In contrast to the NOSTONE study, multiple previous trials showed the benefit of thiazides to prevent kidney stone recurrence. However, most of those trials were small, old, with weak study design and methodology (see our introduction). An important aspect to emphasize is the thiazide dose used in the trials. NOSTONE included lower doses of HCTZ whereas previous trials for kidney stone prevention used a dose of 50 mg or higher, and therefore we still cannot rule out benefit with higher doses. Similarly, indapamide and chlorthalidone are more potent, longer acting, and may have more biologic effect. But, in a chart review (Vigen et al.Int Urol Nephrol 2011) only 35% of stone patients received 50 mg HCTZ, with the rest prescribed lower doses. The low dose HCTZ was speculated to be a result of the practice of using lower doses for hypertension, or lack of knowledge of the results in kidney stone RCTs, or possibly higher doses not being tolerated since these are normotensive patients. Even in this trial there was a suggestion of lower radiologic recurrence at 25/50 mg, though without a clear trend for dose-benefit despite sensitivity analyses. Fans of chlorthalidone and indapamide now also need to clearly demonstrate a benefit (since the higher adverse effects with HCTZ would be even higher with CTD/indapamide).
Another point is the trial duration, around 3 years in NOSTONE, which has been argued by some may not be long enough. However 3 years is a pretty long duration, with a 49% event rate in the placebo, thus more than sufficient to detect any possible benefit. Moreover, this trial had a higher than expected (~ 15% at 12.5 mg, and 25% in other doses as well as placebo group) incidence of low adherence to treatment which could have diluted a potential benefit of treatment. The authors did perform per-protocol analysis to assess if there is a dose effect but results were similar to the intention-to-treat analysis. This also probably reflects real practice, where decreasing adherence over time is not uncommon.
The physiologic basis of thiazide use in kidney stone prevention is the reduction of urinary calcium excretion. This trial confirmed that patients on HCTZ had decreased urinary calcium excretion. However, urine relative supersaturation ratios for calcium oxalate and calcium phosphate were not lower among patients on HCTZ vs placebo. Urinary citrate excretion tended to be lower among patients receiving HCTZ vs placebo, and there was an increase from baseline in urinary oxalate excretion in all groups. The changes in citrate and oxalate excretion may have counteracted the lower urinary calcium excretion with , resulting in no evident differences in relative supersaturation ratios. Similarly, the high fluid and sodium intake during follow-up may have reduced a potential benefit. Additionally, the trial excluded patients on alkali therapies such as potassium citrate, which is known to increase urinary citrate and might actually show benefit in combination with thiazide. This might mean that even if HCTZ has a benefit, it does not have it alone - but possibly as part of a multi-pronged approach (which now needs to be clearly demonstrated).
Conclusion
The NOSTONE trial reported that treatment with HCTZ did not significantly prevent symptomatic or radiologic kidney stone recurrence in patients at high risk for recurrence. This challenges the current practice and suggests that using thiazides alone for kidney stone prevention is not enough. Questions remain to be answered including if thiazides still have a role in some groups of patients or concomitantly with other interventions, and whether they need to be stopped if there is no proven benefit and to avoid side effects.
Summary prepared by
Nikita Pawar
MD, DrNB Nephrology
Nephrologist, Mumbai, India.
And
Rasha Alawieh
IM resident, CT, USA
NSMC Interns, Class of 2023