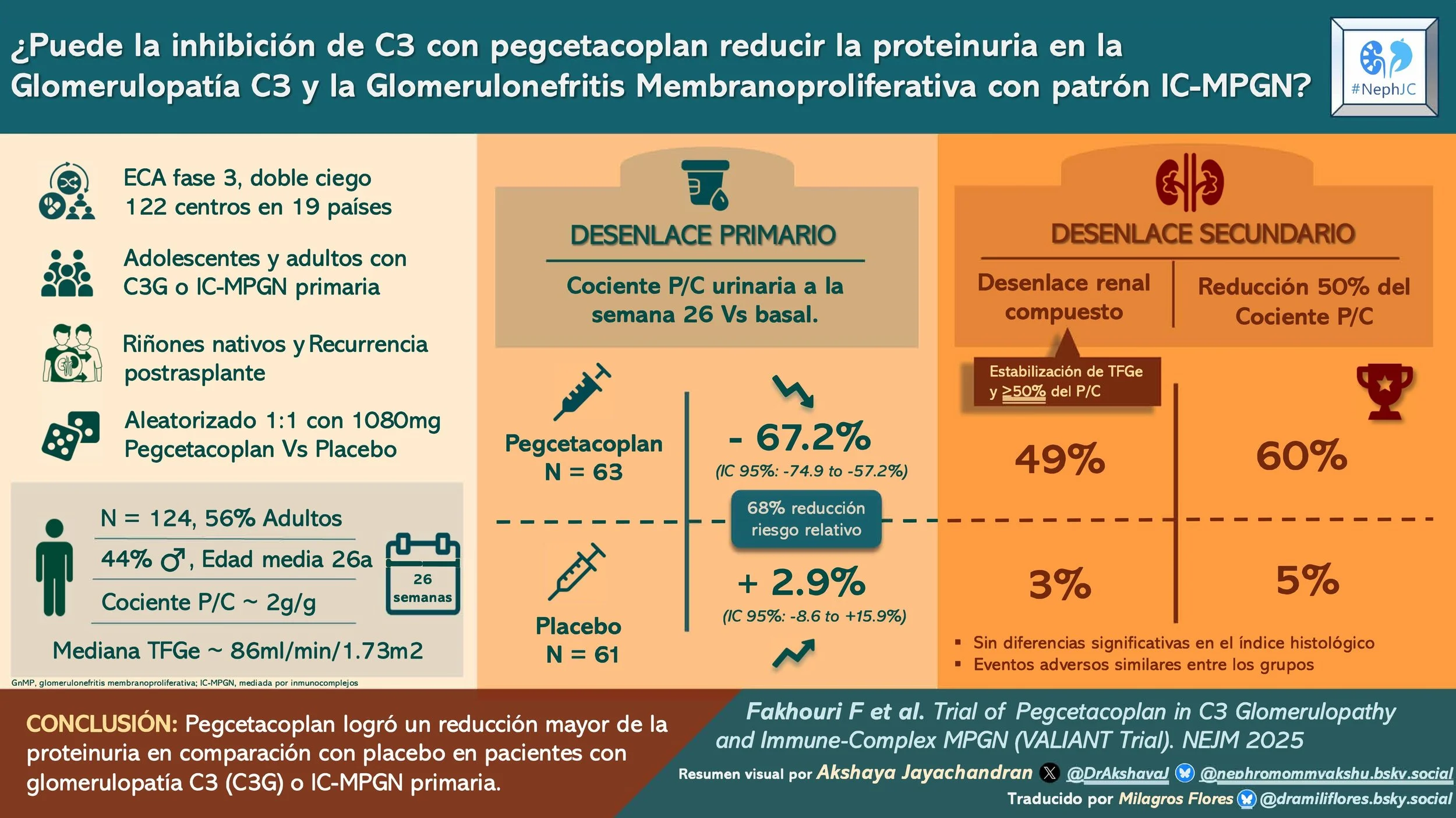
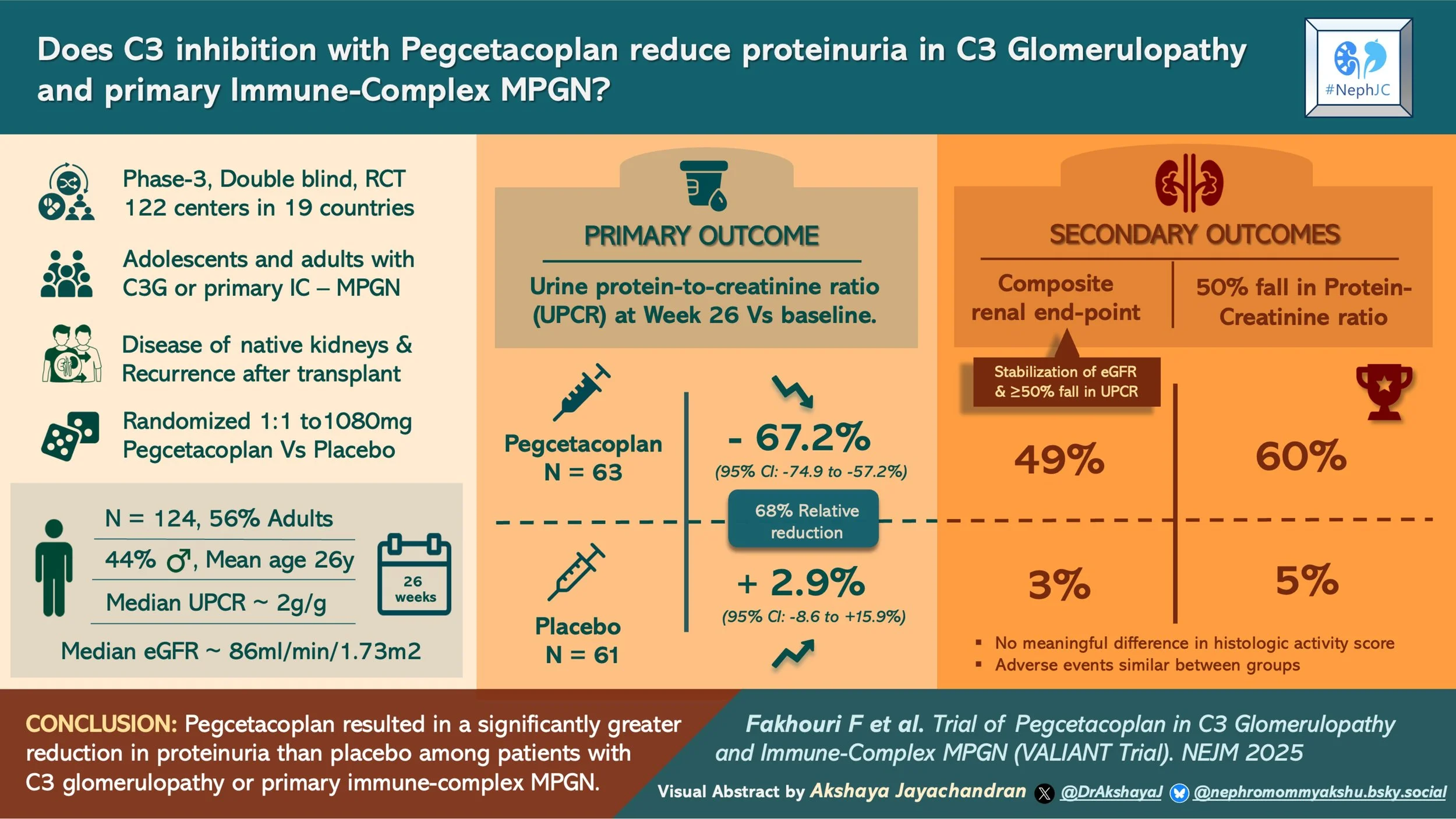
#NephJC Chat
Tuesday, Jan 13th 2025, 9 pm Eastern on Bluesky
N Engl J Med. 2025 Dec 4;393(22):2210-2220. doi: 10.1056/NEJMoa2501510.
Trial of Pegcetacoplan in C3 Glomerulopathy and Immune-Complex MPGN
Fadi Fakhouri, Andrew S Bomback,, Gema Ariceta, Yahsou Delmas,, Bradley P Dixon,, Daniel P Gale, Larry A Greenbaum,, Seung Hyeok Han, Nicole Isbel, Moglie Le Quintrec , Christoph Licht, Antonio Mastrangelo, Masashi Mizuno, Maria Izabel Neves de Holanda, Matthew C Pickering, Giuseppe Remuzzi, Nicole Van De Kar, Marina Vivarelli, Patrick D Walker, Dean Wallace, Daniel Zecher, Cedric Francois, Pascal Deschatelets, Li Li, Zhongshen Wang, Lydia Abad-Franch, Nils Kinnman, Luis López-Lázaro, Johan Szamosi, Carla M Nester; VALIANT Trial Investigators Group
PMID: 41337715
Introduction
Idiopathic immune complex membranoproliferative glomerulonephritis (IC MPGN) and C3 glomerulopathy are rare glomerular disorders. Initially, both were classified into types of MPGN depending on the location of immune deposits: type I (subendothelial), type II (intramembranous), and type III (subepithelial and subendothelial). Classification of MPGN was modified based on underlying pathophysiology and immunofluorescence findings (Sethi et al, Semin Nephrol 2011; Bomback et al, Nat Rev Neph 2012).
Figure from D’Agati et al,KI 2012.
Despite this revised nomenclature, primary immune complex MPGN and C3G cannot be classified into watertight compartments, as a dysregulated alternate complement pathway is demonstrated in both entities (Iatropoulous et al, CJASN 2018; Garam et al, CKJ 2020). The pathology may be driven by genetic factors involving pathogenic variants in complement genes or by acquired antibodies against the complement pathway (i.e. C3 nephritic factor targeting the C3 convertases) (Bomback et al, KIR 2024). Complement disorders are complex with many regulators and amplification loops. Hence, although idiopathic IC MPGN and C3G differ in histology, their clinical presentations are similar.
Within 10 years after diagnosis, up to 50% of C3G patients progress to ESKD, requiring dialysis or transplantation. Furthermore, recurrent C3G leads to allograft loss in up to 60% of kidney transplant grafts (Bomback et al, Kidney Int 2018). There remains a huge unmet need in therapeutics of C3G in the pre and post kidney transplant patient.
Treatment of C3G, till date, consists of RASi to decrease proteinuria and immunosuppression in the form of steroids and mycophenolate mofetil for moderate to severe disease when proteinuria exceeds >2g/d or with declining kidney function (KDIGO GN guidelines 2021,Caravaca-Fontan et al. CJASN 2020). MMF and steroids work better in those with autoantibody driven disease but are less effective in those with genetic mutations. Additionally, there is a risk of relapse of disease activity after withdrawal of immunosuppression. In those without a response, eculizumab can be considered based on retrospective studies of complement blockade, but the clinical response to the C5 inhibitor is limited (Welte et al, BMC Nephrol 2023; Quintrec et al, AAJKD 2018). Recently, iptacopan, which targets the alternative complement pathway by binding Factor B and regulating C3 cleavage, was shown to have efficacy in C3G in the APPEAR-C3G trial (Kavanaugh et al, Lancet 2025). Consequently, the therapeutic target has been repositioned upstream in the cascade, from C5 to C3, to influence the amplification and effector generation processes (C5a and MAC).
The next generation of complement inhibitors, pegcetacoplan, belongs to a family of cyclic peptide inhibitors termed ‘compstatins.’ Pegcetacoplan acts by inhibiting C3, C3b, and C3 convertase (C3bBb), preventing formation of C3b and C3bBb, and thereby inhibiting complement activation through the classical, lectin, and alternative complement pathways. It was initially approved for paroxysmal nocturnal hemoglobinuria (PNH), where pegcetacoplan showed a better hematological clinical response in a head-to-head comparison with eculizumab in the PEGASUS trial (Hillmen et al, NEJM 2021). In a phase 2 trial (Dixon et al, KIR 2023) pegcetacoplan demonstrated efficacy in decreasing proteinuria when used in patients with C3G in native kidneys. Moreover, the NOBLE phase 2 trial (Bomback et al, KI Reports 2024) demonstrated the efficacy of pegcetacoplan in decreasing complement deposition on kidney biopsies along with reduced proteinuria and stabilization of eGFR when used in patients with post transplant recurrence of C3G or primary immune complex MPGN.
Based on these results, the VALIANT trial was designed to study the effect of pegcetacoplan in both native and transplant kidneys with C3G or IC MPGN. Can proximal C3 inhibition with pegcetacoplan provide better outcomes in patients with C3G?
Therapeutic targets in C3G, Figure from Kim S et al. KN 2026
The Study
Methods
This phase 3, double-blind, randomized, placebo-controlled trial was conducted at 122 centers in 19 countries. There was a 10-week screening period, a 26-week randomized control period, and an additional 26 weeks of observation for the open-label group with a 120-week VALE extension to monitor long-term complications. There was also an 8-week follow-up for those who did not enroll in the long-term follow-up. Randomization 1:1 to pegcetacoplan versus placebo was computer-generated and stratified by transplantation status and baseline biopsy availability.
Figure S1. VALIANT study design from Fakhouri et al. NEJM 2025
Study population
The participants included eligible adults and adolescents (>12 years and weight >42 kg) with biopsy-proven C3G or IC MPGN, including both native kidneys and post-transplant recurrence. Biopsies were not required in adolescent patients who had biomarkers suggesting active disease.
Interventions
Patients weighing ≥ 50 kg received 1080 mg of subcutaneous pegcetacoplan twice a week. There were weight-adjusted dosing schedules for adolescents < 50 kg. Rescue therapy was permitted (e.g., high-dose glucocorticoids and/or C5 inhibitors) for major creatinine rise attributed to the disease.
Standard medications
Standard medications (steroids at prednisolone equivalent <20 mg per day, MMF, RASi, and SGLT2i) were allowed if on stable doses ≥12 weeks prior to randomization and 4 weeks prior to biopsy and continued the same even after randomization.
Vaccination
Vaccination against encapsulated organisms—Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae (type B)—was done ≥14 days prior to randomization.
Outcomes
The primary outcome was the change in UPCR at week 26 compared to baseline in the study arm compared to the placebo arm. Secondary endpoints at week 26 were tested hierarchically and included a composite renal endpoint and histological activity score. All patients underwent a kidney biopsy at the end of the randomized controlled period for evaluation of this histological scores by a central pathologist.
Statistical analysis
The analysis was done after completion of 26 weeks of treatment by intention-to-treat analysis. Safety analyses were done in all who received ≥1 dose. Longitudinal assessments for changes from baseline in continuous endpoints were analyzed by a mixed-effects model for repeated measures. The binary endpoints were analyzed using the logistic regression model (authors presented relative risks for interpretability). The secondary endpoint of the activity score of the C3 glomerulopathy histological index (Bomback et al, KI 2018) was calculated by analyzing the covariance model. The fixed-sequence hierarchical testing strategy was used to control the type I error across key secondary outcomes. Based on the findings from the DISCOVERY trial (Dixon et al, KI Reports 2023), the sample size was set at approximately 70 in each group, achieving 90% power with a one-sided significance level of 0.025.
Missing data and events that interfered with outcomes assessment were handled using prespecified methods detailed in the protocol and statistical analysis plan, including sensitivity analyses that tested how robust the results were to non-random missing data (tipping-point analyses).
Funding
Apellis Pharmaceuticals, the manufacturer of pegcetacoplan, funded the study. Apellis designed the study, funded and conducted trial operations, provided the drug, and was involved in data collection, statistical analysis, and interpretation per protocol. Several authors are employees of Apellis, as shown in the authors' affiliation list. Other authors are academic investigators, many with declared financial relationships with Apellis.
Results
Over a period of 2 years, 261 patients were screened, and 124 were randomized (63 to pegcetacoplan, 61 to placebo). A total of 59 patients in each arm completed the 26-week randomized controlled study.
Figure S3. Patient disposition- patient with ongoing study treatment had completed the week 26 assessment, but had not started the open-label period, from Fakhouri et al. NEJM 2025
With respect to the demographics almost half of the cohort were adolescents (44%). Mean age among adults was higher in the pegcetacoplan group. There was a slight female predominance overall (~56%). Greater than 70% of the patients were White, and 14% Asian. The majority of the included patients had C3G (77% overall), and IC MPGN represented one quarter. A small proportion with post-transplant recurrence was evenly distributed (~7%). Proteinuria was high in both groups, but numerically higher in the pegcetacoplan arm, eGFR was lower in the pegcetacoplan group (78 vs 91 ml/min/1.73 m2). Regarding background therapy, >90% patients were on RASi, ~10% on flozins, 70% had immunosuppression (40% on glucocorticoids). Around 94% in both groups completed the treatment for 26 weeks with 80% adherence.
Table 1 from Fakhouri et al. NEJM 2025
Primary efficacy endpoint
At week 26, the pegcetacoplan group showed a change in geometric mean UPCR of −67.2% (-74.9 to −57.2) compared to placebo with a +2.9% [95% CI, −8.6 to 15.9]. This amounted to a relative risk reduction of 68.1% with pegcetacoplan (95% CI, 57.3 to 76.2; P<0.001). Proteinuria reduction started as early as 4 weeks after therapy initiation and was sustained throughout the randomized period. Additionally, proteinuria reduction was consistent across all subgroups.
Figure 1 from Fakhouri et al. NEJM 2025
Figure S5 from Fakhouri et al, NEJM 2025. Change from baseline to week 26 in UPCR
Secondary endpoints
Composite renal endpoint (eGFR stabilization + ≥ 50% UPCR reduction) at week 26 was higher in the pegcetacoplan group (49% vs 3%), with a relative risk of 14.4 (95% CI, 3.7 to 56.9). (Table 2, fig. S6)
≥ 50% UPCR reduction at week 26 was higher in the pegcetacoplan arm 60% vs 5%, with a relative risk of 12.0 (95% CI, 4.0 to 36.1). (Table 2, fig S7).
Table 2 from Fakhouri et al. NEJM 2025
In the post hoc analyses, pegcetacoplan treatment was associated with favorable proteinuria category shift. The proportion of patients with UPCR <1g/g increased from 8% to 51%, and those with nephrotic-range proteinuria (≥ 3g/g) decreased from 38% to 14% with pegcetacoplan.
Figure S8. Proteinuria shift from baseline to week 26 in patients receiving
pegcetacoplan or placebo, from Fakhouri et al, NEJM 2025
eGFR stabilization component
Stable/ improved eGFR (≤15% decline) was seen in 68% of patients on pegcetacoplan vs 59% of those on placebo (reported alongside composite endpoint).
Biopsy/ histology and downstream hierarchy implications
Among 69 patients with biopsy samples, the C3G histologic index activity score change did not differ significantly between groups at week 26 (Table 2, fig S9). Because of hierarchical testing, subsequent endpoints were not tested for significance.
Descriptive biopsy signals were strong. A decrease in C3 staining at week 26 was more frequently seen in the pegcetacoplan group 74% vs 12%; staining fell to 0 intensity in 71% vs 9% respectively.
Figure 2. C3 staining in kidney biopsies, from Fakhouri et al. NEJM 2025
Kidney function (eGFR)
Least-squares mean eGFR change from baseline to week 26 was in favour of pegcetacoplan with a difference +6.3 mL/min/1.73m2 (95% CI 0.5 to 12.1).
Figure S10. Key secondary endpoint of change in eGFR from baseline to week
26, fromFakhouriet al. NEJM 2025
Complement biomarkers
Directional biomarker shift was consistent with proximal complement blockade: serum C3 appeared higher, and soluble C5b-9 was lower over time in pegcetacoplan arm.
Figure S11. Complement biomarker concentrations over time, from Fakhouri et al. NEJM 2025
Safety
The adverse event profile was comparable between the two groups, including severe adverse events (Table 3 below). In the pegcetacoplan group, one patient developed COVID, influenza, and pneumonia. One trial participant in the pegcetacoplan group (and also receiving MMF) died in the context of developing severe COVID pneumonia with respiratory failure on a background of diabetes, obesity, and COPD.
Table 3 from Fakhouri et al. NEJM 2025
Discussion
C3 glomerulopathy is a disease in which terminal complement inhibitors, like C5 inhibitor eculizumab (Quintrec et al., AJKD 2018), the C5a inhibitor avacopan (Bomback AS et al. CJASN 2025), and the Factor D inhibitor danicopan (Nester C et al. Am Jour Neph 2022), have shown disappointing results. However, 2025 has been a favorable year for C3G disease as the focus shifted from C5 blockade to C3 inhibition. The first success was the Factor B inhibitor, iptacopan (Kavanaugh et al, Lancet 2025) which showed a significant reduction in proteinuria in patients with C3G and depicted the urgent need for medications acting at the C3 level. The year ended at an all time high for C3G, with this very VALIANT trial, which provides evidence that proximal complement inhibition with pegcetacoplan leads to meaningful reductions in proteinuria, eGFR, and changes in histology in C3G and primary immune-complex MPGN. The observed treatment effects align with the ones proposed by the C3G Trial Endpoints Workgroup (Nester, C. et al. CJASN 2024).
In VALIANT, pegcetacoplan resulted in a substantial and early reduction in proteinuria, with a large proportion of participants achieving both ≥50% reduction in UPCR and stabilization of eGFR over 26 weeks. These findings are clinically relevant given prior observational data demonstrating proteinuria as the strongest predictor of progression to kidney failure in C3G (Fontan et al, NDT 2022). Even a 50% reduction in proteinuria was associated with improved kidney survival, while sustained reductions to <300 mg/day were linked to a lower risk of ESKD. Pegcetacoplan was efficient in all subgroups irrespective of age, sex, race, prior transplant status, or whether they were on immunosuppressant medication.
VALIANT builds directly on prior evidence supporting upstream complement inhibition in C3G. In the phase 3 APPEAR-C3G trial, iptacopan, a selective factor B inhibitor targeting the alternative pathway, demonstrated significant reductions in proteinuria and improved renal composite outcomes compared with placebo. VALIANT extends this therapeutic paradigm by targeting C3 itself, thereby suppressing complement activation across all pathways and directly inhibiting the central amplification loop (Kim et al, ASN Kidney News 2026).
Table 1 from Kim et al, ASN Kidney News 2026.
A notable aspect of VALIANT is the substantial inclusion of adolescents, who comprised nearly half of the trial population. C3 glomerulopathy frequently presents in childhood or adolescence and is associated with a prolonged disease course and high lifetime risk of kidney failure and transplant recurrence. Pegcetacoplan demonstrated robust and consistent reductions in proteinuria among adolescents, with numerically greater responses than those observed in adults. Moreover, VALIANT’s adolescent-specific design, including weight-adjusted dosing and the avoidance of mandatory repeat kidney biopsies, illustrates the feasibility of conducting appropriate, scientifically driven trials in this population. The inclusion of patients with primary immune complex MPGN and those with post transplant recurrence of disease, although small in number, widens the therapeutic potential of the drug in these nebulous entities of complement dysregulation which have no as yet defined diagnostic criteria or approved treatments.
Histologic parameters were analysed by the C3 glomerulopathy histological index activity score (Bomback et al, AJKD 2018). Although this score is not validated in many populations (Caravaca et al, AJKD 2021; Miroglu et al, CKJ 2020) a higher chronicity score may be associated with a higher risk of disease progression. The current study evaluated the histological score by hierarchical testing and although there was no significant change in the activity score, it’s somehow heartening to nephrologists to see a signal for decrease in C3 staining, which is otherwise associated with a worse kidney outcome not just in C3G/IC MPGN but also in other glomerulonephritis. Although the study planned to do kidney biopsies in all patients at the end of the 26 week randomized period, only 63 evaluable biopsies were available at the study end, and hence the results need to be taken with a pinch of salt.
As C3 glomerulopathy and IC MPGN may be driven by genetic predispositions, it would have been interesting to know the response to pegcetacoplan in the subgroup of patients with genetic predisposition or autoantibodies versus those without such risk factors. Additionally, such patients may require prolonged therapy, safety and efficacy data, which may be available once the open label VALE extension data is released.
As therapeutics in nephrology evolve from broad spectrum immunosuppression to targeted pathophysiology driven agents, it is yet to be seen if pegcetacoplan is able to provide us a long term therapy for stabilisation of eGFR and prevention of kidney failure in this devastating disease of the young. The good news is that 2025 marks the dawn of complement inhibitors. With FDA approvals of both iptacopan and pegcetacoplan in 2025, we are excited to see what the future holds for this enigmatic disease.
Conclusion
Pegcetacoplan, a C3 and C3b inhibitor, resulted in a significant reduction in proteinuria versus placebo in patients with C3 glomerulonephritis or primary immune-complex MPGN.
Summary by
SaiVani Yellampalli
Assistant professor of Nephrology
Santhiram Medical College
Reviewed by
Cristina Popa, Milagros Flores,
Pallavi Prasad, Brian Rifkin