In 2010 Matt Sparks started the longest tradition in Nephrology Social Media, The Top Nephrology Stories of the Year. In 2017 this project moved from Renal Fellow Network to NephJC, where it has been hosted ever since. Here are all 6 of the NephJC Top Stories (The 2017 post has links to the 7 prior editions on the Renal Fellow Network for you nostalgic types).
And with no further blah blah blah, here are the top nephrology articles of 2022…
EMPA-Kidney: The Flozin Finale?
It's been a magnificent 7 years running for flozins on our annual shortlist and they’re here twice in 2022, including their second outright win in this Hall of Fame worthy trial. EMPA-KIDNEY (Herrington et al, NEJM 2022) was a clean RCT of empagliflozin 10 mg versus placebo in adult patients at risk of progressive CKD. They randomised 6,609 participants, and pushed the envelope beyond CREDENCE and DAPA-CKD in multiple ways; over half did not have diabetes, over a third had eGFR <30, and almost half had urine ACR <300mg/g (<34mg/mmol). It was also the largest IgA nephropathy RCT yet, with 817 patients included. As with all our favourite flozin trials it had to be stopped early for efficacy, with the composite primary outcome of kidney disease progression or cardiovascular death occurring in 432/3,304 (13.1%) patients in the empagliflozin group versus 558/3,305 (16.9%) patients in the placebo group (HR = 0.72, 95% CI = 0.64 to 0.82, P<0.001), giving a 28% risk reduction with empagliflozin over mean follow-up of 2 years. More patients discontinued placebo due to intolerability than empagliflozin, and safety profile was consistent with results of the concurrently published flozin meta-analysis in the Lancet (also a must read for nephrologists - I was particularly reassured by the DKA rate in those without diabetes of 1 in 15,592). Efficacy and safety in proteinuric CKD down to eGFR 20 is the easy win in EMPA-KIDNEY, and implementation in this group is now key, though we also look forward to post-hoc analysis of 254 patients with eGFR as low as 15-20. The less certain question is flozination in patients with CKD with low/no proteinuria (and without diabetes or heart failure as other indications, obviously). Events accrue more slowly in these patients so they only experienced a tiny fraction of the events in EMPA-KIDNEY, and their results for the primary outcome were neutral. However, slope analysis in this subgroup showed slowing of eGFR decline with empagliflozin, and hopefully in 2023 we’ll see meta-analysis with patients from the heart failure trials to further examine the key outstanding question of degree and timing of kidney benefit from flozins in non-proteinuric CKD.
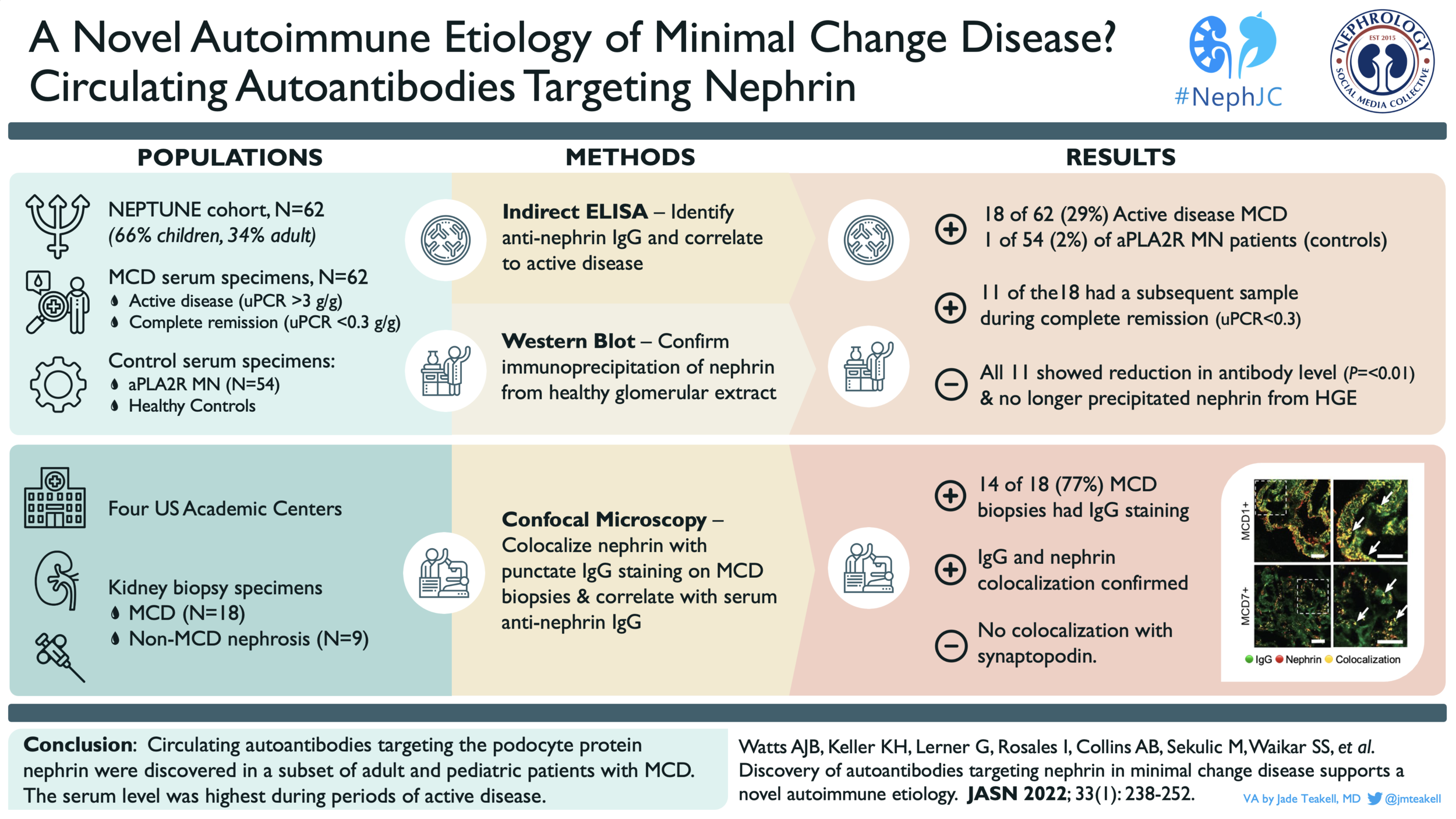
Anti-Nephrin antibodies in MCD
If only pediatric nephrologists were allowed to vote for the #NephJC Top Nephrology Stories of 2022, then Anti-Nephrin Antibodies in Minimal Change Disease (MCD) would have been the clear winner. Throughout our training and careers we see child after child with MCD that comes on suddenly and turns off just as quickly with immunosuppression. We’ve been asking ourselves “why” for over 50 years. Dr. Astrid Weins and her research group may have finally found an explanation. They took their long standing observation of a delicate punctate staining for IgG in some patients with MCD and asked themselves, “what is this?” They then confirmed that this “dusting of IgG” was due to autoantibodies against nephrin in a subset of pediatric and adult patients with MCD and showed that these antibodies correlated with disease activity, including at least one patient post-transplant. (#NephJC summary here) Are we done now? Do we know what causes MCD and how to treat it? Of course not. This is likely one of many causes of MCD but an important one. Next we need to understand if the presence of these antibodies impacts treatment response or transplant recurrence risk to truly start to apply a personalized medicine approach to these patients. Flozins are great and all, but Anti-Nephrin Antibodies in MCD was robbed of the title.
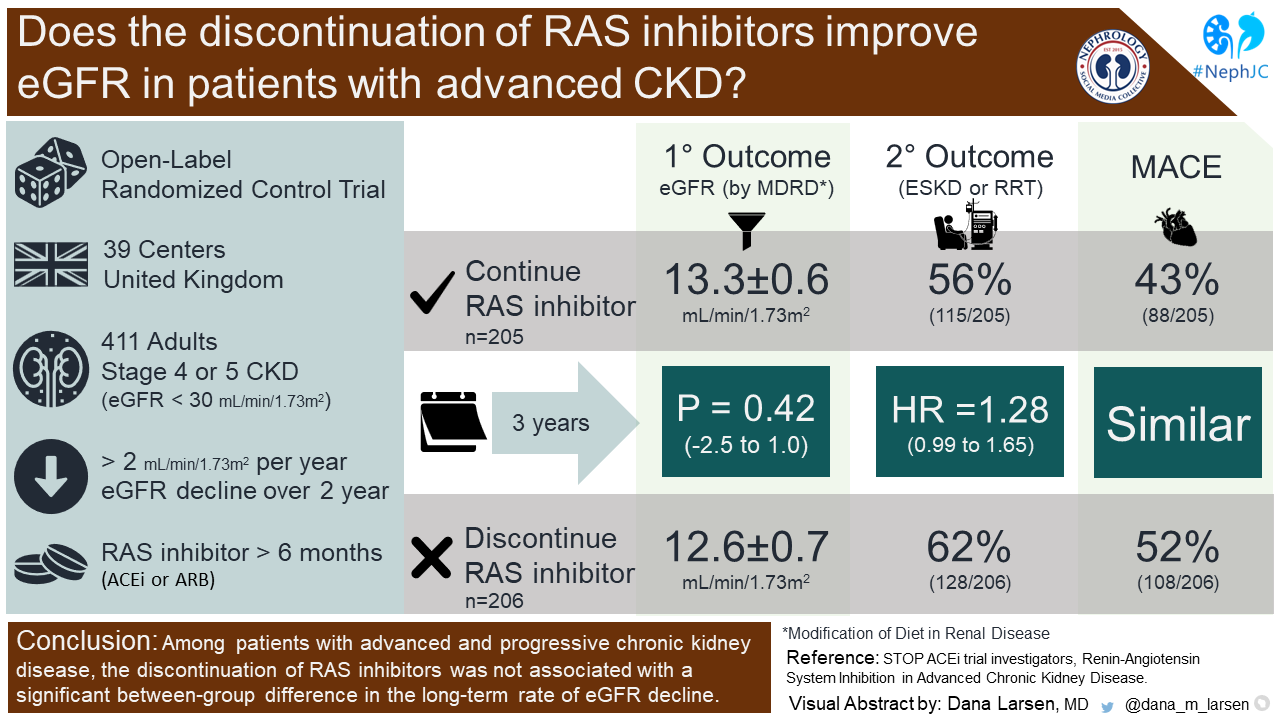
STOP-ACEi: Or rather, Don’t Stop ACEi in CKD!
RAS inhibitors are a foundational treatment of patients with CKD and proteinuria (Lewis et al. NEJM 2001). They are also quite beneficial in decreasing devastating cardiovascular outcomes, a major risk in CKD. In advanced CKD, however, one might consider withholding RAS inhibition to prevent hyperkalemia, or get a “bump” in eGFR to prolong time off dialysis. STOP ACEi (Bhandari et al. NEJM 2022) was a multi-center, randomized, open-label trial that examined the impact of continuation of RAS inhibitors on the eGFR in advanced CKD G4-5. 411 patients were randomized at 39 centers in the United Kingdom. RAS inhibitors were defined as ACEi or ARB. At 3-years, the least-squares mean eGFR in the continuation group was 12.6 ± 0.7 and was 13.3 ± 0.6 in the discontinuation group (p = 0.42). A bit surprising based upon what we’d expect from hemodynamic glomerular changes upon stopping RAS inhibitors (no eGFR “bump” detected). In addition, ESKD or RRT at 3-years occurred in 128 (62%) patients in the discontinuation group and 115 (56%) patients in the continuation group (HR 1.28, 95% CI 0.99-1.65), again no signal of benefit in the discontinuation group. Of the 490 serious adverse events, no significant differences were reported between groups, not even hyperkalemia. The STOP-ACEi trial did not find any benefit by stopping ACEi (or ARBs) in advanced CKD. Indeed, the discontinuation arm had a higher risk of needing KRT, as well as a higher risk of CV events. Thus, the findings of the STOP ACEi trial overturned the results of the previous study (Ahmed et al. NDT 2010). The cardioprotective benefits of RAS inhibitors in advanced CKD have been previously demonstrated in large observational studies (Qiao et al, JAMA Intern Med 2020; Fu et al, JASN 2020), which does further support continuation of RAS inhibitors even in advanced CKD. However, it is important to note that the STOP ACEi trial was not adequately powered to examine the effect on mortality or cardiovascular outcomes. The open-label nature of this study may have contributed to bias. Additionally, there was a relatively low proportion of patients included who had a history of congestive heart failure. In conclusion, stopping RAS inhibitors in advanced CKD at an arbitrary eGFR threshold appears unnecessary.
Xenotransplantation
We discussed this in 2021 (scroll to the sixth story here), so why the redo? Well, all we had in 2021 were the news stories, the underlying science, and a lot of speculation. Now we have some papers - so let’s dive in. Last year we discussed some of the initial attempts (chimpanzees to pigs) and initial hurdles (alpha-gal, porcine endogenous retroviruses [PERV] and the differences in major histocompatibility antigens). These hurdles have been overcome in the proprietary pigs from Revivicor (now part of United Therapeutics) which lead to the kidney and heart transplants reported in the media last year. But how did they actually perform in the decedent model? The first publication came from the folks at the University of Alabama (NephJC Summary) where the kidneys were transplanted from a 350 lb genetically engineered pig into a brain dead decedent (Mr. Parsons, after whom the UAB program has labeled this as the ‘Parsons model’). There was no hyperacute rejection, the kidneys produced urine - but the creatinine did not come down, for unclear reasons - possibly tubular necrosis in the brain dead pathophysiological milieu. The other kidney transplants (two this time) were performed (with the same pig kidney source, but a slightly different knockout model) at NYU Langone (Montgomery et al, NEJM 2022). Among the differences were the use of a ’thymokidney’ in which a thymic extract from the pig was implanted under the renal capsule a few weeks prior. In these two cases, the kidneys did keep producing urine (till the end of the study at 54 hours) and more importantly the creatinine decreased, with kinetic GFR calculations suggesting kidney function recovering nicely. But he native kidneys had not been removed (unlike at UAB) so it is hard to say how much the xeno-kidneys contributed, The kidneys (biopsied several times during the 54 hours) did not show any concerning features, there were no increases in any alpha-gal antibodies, inflammatory markers, or presence of PERVs. Until 54 hours that is. Which is the main shortcoming or limitation of these studies - the model itself (brain dead decedents) and the short follow up. Are we ready for human trials? Well, using the same model as at UAB, folks at the University of Maryland performed a pig to human heart transplant in a 57 year old man who was not a candidate otherwise for a transplant (Griffith et al, NEJM 2022). The heart worked well, allowing the recipient to be weaned off ECMO, but failed inexplicably at 7 weeks, and the recipient passed away at day 60. The key aspects of these experiments are discussed in an accompanying editorial (Pierson, NEJM 2022) which is well worth reading. Ethical issues, including in whom should these be tested next remain an important consideration (Cooper et al, JASN 2020). We live in interesting times.
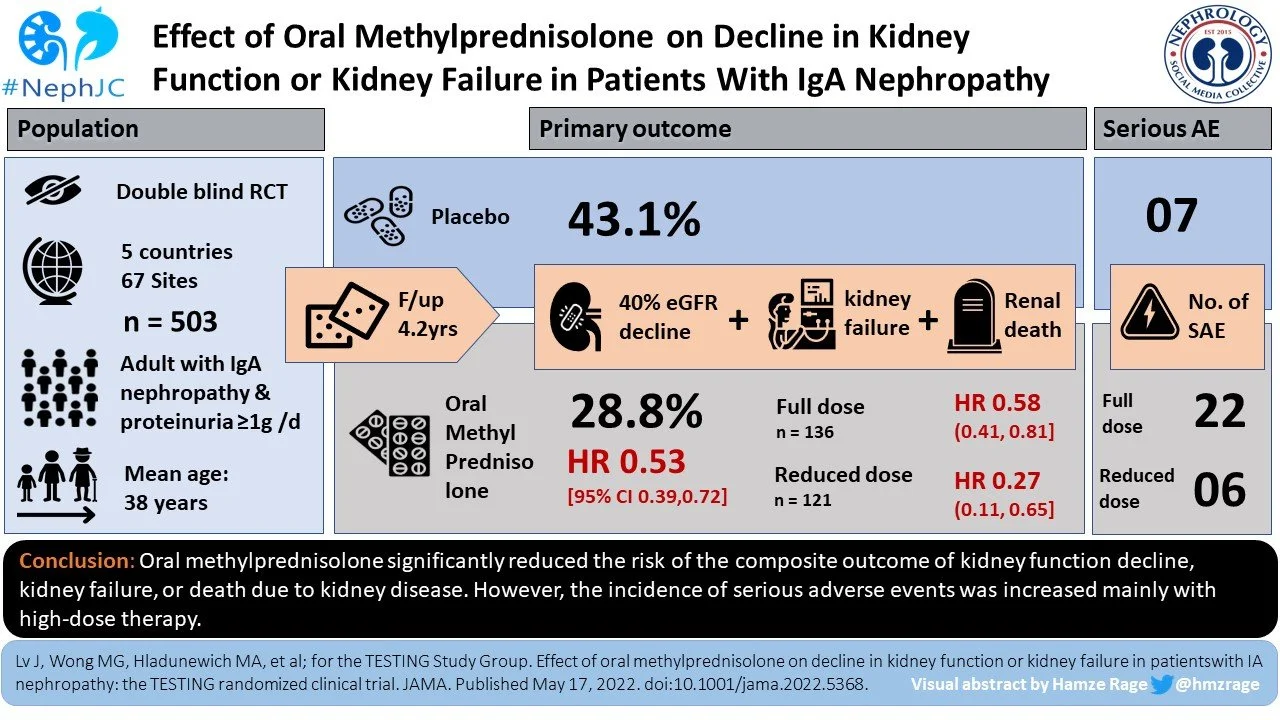
TESTING-2: Steroids in IgA Nephropathy
Steroids and IgA Nephropathy are the Rachel and Ross of nephrology. They get together, they break up, they get together, they break up, and 2022 was the year they finally got married. The Nuptials were presented in JAMA with the publication of “Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial” or what we call TESTING-2. This is TESTING’s second act. The first came in 2017 when steroids were doing great but the trial was halted due to a serious imbalance in adverse events: 28 with methylprednisolone versus 4 with placebo. Infectious adverse events were 13 to zero. But the TESTING team went back to the drawing board and rewrote the protocol with a reduction in the steroid dose from 0.6-0.8 mg/kg/day to 0.4 mg/kg/day. And they added trimethoprim/sulfamethoxazole as pneumocystis prophylaxis. This resulted in a clean win with a HR of 0.53 for the primary outcome (death dialysis, or 40% decrease in GFR) over 4.2 years. The report included all adverse events (full and reduced dose) and showed the same imbalance in adverse events as in the initial publication, but importantly after reducing the doses serious adverse events were much rarer (16% with full dose versus 3% with reduced dose). In conclusion, both TESTING publications showed clear efficacy for steroids and with the dose modification it appears the drug is safe-ish. There is a lot of interest for new therapies for IgA nephropathy but steroids look to be effective and newcomers should be tested against this staple rather than placebo to prove their worth.
CAR T brings hope in Refractory Lupus
The last 5 years has provided many advances in the treatment of lupus. However, nothing holds a candle to the potential use of CAR T cells to treat this challenging disease. 2022 saw the publication in Nature Medicine of a series of 5 patients (no placebo group) with treatment refractory lupus who were admitted to the hospital for a 10+ day period for CAR T cell therapy. First, what in the world are CAR T cells? This is usually something given to a patient with leukemia and a nephrologist only gets involved during an AKI aftermath. CAR stands for chimeric antigen receptors. CARs allow T cells to recognize any cell-surface antigen independently of antigen processing or MHC-restricted presentation. To do this, a patient's native T cells are removed from the body and an artificially constructed antibody-derived receptor is placed on the T cells allowing them to recognize whatever cell type desired. In this case it is CD-19, a B cell marker. Importantly, the antibody-derived receptor is also outfitted with powerful co-stimulatory molecules allowing the infused CAR T cells to become a deadly assassin destroying any cell displaying CD-19, thus ridding the body of all B cells. This has proven effective in B cell leukemias refractory to standard therapy, but significant side effects, cost, and laborious administration, limit widespread use. Importantly, cytokine release syndrome and neurotoxicity are feared side effects which need to be considered. This Nature Medicine study showed miraculous results. I usually refrain from such hyperbolic language, especially in a non randomized study, but the results are impressive. To be clear, the use of CAR T cells in leukemia started with non-randomized studies so there is precedent. The 5 patients who participated in the study, all underwent a 1 month long process that included a 14-day stay in the hospital. The patients all had lupus which was refractory to the host of standard medications (you name it, they got it). However, it must be noted that they also received fludarabine and cyclophosphamide in addition to CAR T cells as part of the protocol. All 5 of the patients had improvements (when I say improvement, I mean everything went to normal) in SLEDAI-2K scores, proteinuria, anti-dsDNA, C3, C4, and other auto-antibodies at 3 months. Importantly, none of the patients developed high-grade cytokine release syndrome. Oddly enough, 1 patient did develop minimal change disease that quickly subsided with steroids. We still have a long way to go before this becomes a mainstream therapy. We need to know if the results are durable. Will the side effect profile be acceptable, especially when given to more patients. Lastly, we need to simplify the administration process so it doesn’t require admission to the hospital. I can’t wait to see how this story develops in 2023.
Return of live conferences
After 2 years of attending conferences from your living room, 2022 marked the return of live meetings. Absence really does make the heart grow fonder. Early morning plenaries. Late nights at the Hyatt lobby bar. Blisters. Serendipitously watching a fabulous talk and learning something new. Running into old friends in the hallway. Standing by your poster nervously waiting for someone to talk to you. Meeting your Twitter friends in real life for the first time at the #NephJC party. Having palpitations as you wait to give your talk. Seeking out exhibit hall coffee. Having dinners with friends from fellowship. Enjoying time away from the kids (did I say that out loud?). Trying to run from one end of the conference center to another to catch two talks in the same session you want to hear. Crowding into the late-breaker session to hear the future of nephrology. Being reminded that this is your field for a reason and these are your people. See you next year.
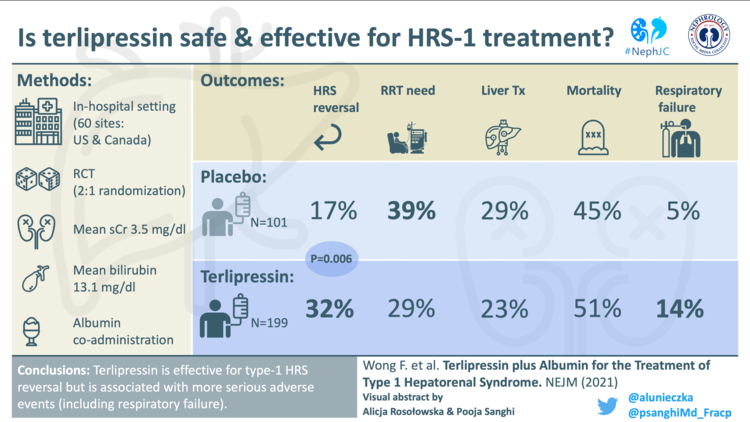
Terlipressin gets the FDA nod
The Europeans have used it for decades, and trial after trial shows clear efficacy over placebo in reversal of hepatorenal syndrome type 1 (HRS-AKI), including impact on hard patient-oriented outcomes like need for acute dialysis. So why has approval of terlipressin taken so long? You get a hint towards the answer when you consider that the largest ever RCT in HRS-AKI (Wong et al, CONFIRM, NEJM 2021) which examined the efficacy and safety of terlipressin, didn’t make our Nephrology Top Ten list in 2021. This was probably because CONFIRM didn’t alter the status quo; despite meeting the primary endpoint for reversal of HRS-AKI of 32% with terlipressin versus 17% with placebo, the FDA looked at the 30 day mortality due to respiratory failure of 8.5% in the terlipressin arm versus 1% with placebo and initially denied approval in September 2020. Excess iv albumin was hoped to be a major and modifiable cause. However this is far from clear cut for multiple reasons, including subsequent analysis (Wong et al, AP&T 2022) which did not show an association between pre-randomisation albumin dose and the risk of respiratory failure. Even pooling the three large placebo-controlled North American RCTs (CONFIRM, REVERSE and OT-0401) for N=598 patients there still is not power for mortality, though 48.7% with terlipressin versus 48.2% with placebo did not look like the “favourable trends” the FDA had stated they wished to see (figures from FDA Terlipressin Advisory Committee Briefing Document, table 33)
So why the U-turn from the FDA in 2022? It is not clear. Most likely they changed their mind in the hope that mitigation strategies based on the learning from the trials can now prevent repetition of some of the bad outcomes in clinical practice, which is reasonable given respiratory failure was poorly recognised as a potential problem prior to CONFIRM. Close attention to oxygen saturations and fluid status is emphasized, and withholding terlipressin if hypoxia is present. Further strategies include extreme caution in populations with much worse benefit to risk ratios, namely acute on chronic liver failure grade 3 or those with starting serum creatinine >5 mg/dL (>442 µmol/L). One unblinded, 71 patient RCT (Cavallin et al, Hepatology 2016) showed lower adverse events with an infusion instead of bolus regime of terlipressin which could also mitigate risk, though disconcertingly serious adverse events leading to drug withdrawal were still 21% in the infusion group (versus 43% in the bolus group). Ultimately, prognosis in hepatorenal syndrome is appalling and therapeutic options are limited, so it is positive that the US now has an FDA-approved treatment. Terlipressin undisputedly has much more efficacy than midodrine-octreotide, but with real vasoconstrictors on the floor come real adverse events, to which the entire international community are now more alert. Post-market surveillance safety reporting, along with thoughtful patient selection and monitoring, will be vital as terlipressin becomes available - missteps are literally fatal.
Flozins DELIVER in HFpEF
SGLT2 inhibitors, aka flozins, work for everything and everyone. Almost. They reduce CV outcomes in people living with diabetes and high cardiovascular risk, reduce kidney failure in those with diabetes, reduce kidney failure in those without diabetes but with just proteinuria, and reduce CV outcomes including death in those with heart failure and reduced ejection fraction (HFrEF). Among the few populations left in whom we did not (yet) have hard evidence of benefit are those with CKD but no diabetes or proteinuria, and those with heart failure but with preserved kidney function (HFpEF). One may count SOLOIST-HF (Bhatt et al, NEJM 2021) as a semi-win in this area, but the dedicated data from EMPEROR-Preserved (Anker et al, NEJM 2021) was definitely disappointing, with the primary outcome being driven by the 27% relative risk reduction in hospitalization for heart failure (HHF), with a small, albeit insignificant reduction in CV death (HR 0.91, 95% CI 0.76 - 1.09) - and no difference at all in all-cause mortality (HR 1.00). In this space landed deliverance from the DELIVER trial (Solomon et al, NEJM 2022). At an N of 6,263, this is the largest trial in HFpEF, and included participants with heart failure, but an ejection fraction of more than 40% and an elevated brain natriuretic peptide. Diabetes was not necessary (~ 45% had diabetes in the final tally) - and of interest to us, included participants down to an eGFR of 25 ml/min/1.73m2. The effect of dapagliflozin on the primary outcome was again driven by the 23% HHF relative risk reduction. The reduction in CV death (HR 0.88, 95% CI 0.74 - 1.05) and all-cause mortality (HR 0.94, 95% CI 0.83 - 1.07) was tantalizingly close. A bit more impressive was the secondary composite outcome of CV death and worsening heart failure events (HR 0.77, 95% 0.67 - 0.89) and the significant benefit in patient reported outcomes (the change in the KCCQ symptom score). From a renal perspective, the subgroup analysis demonstrated similar benefits in those with GFR >/< 60 ml/min/1.73m2. As a harbinger of things to come with EMPA-KIDNEY, the benefit in the kidney composite outcomes - which were infrequent at 1.1 events per 100 patient-years - was not seen (McCausland et al, JAMA Cardiology 2022). However, there was a benefit seen in slowing the GFR decline (difference, 1.4; 95% CI, 1.0-1.8 mL/min/1.73 m2 per year). Lastly (again, a parallel with the post EMPA-KIDNEY example), a prespecified meta analysis (Vaduganathan et al, Lancet 2022) packaged the data from the 5 major HF trials (HFpEF and HFrEF). They overall show a consistent benefit in all major outcomes, including CV death and all-cause death, with no evidence for heterogeneity. That’s the lesson for the nephrology community as well - let’s look at the totality of evidence rather than individual trials, especially as we enter the low risk and low event rate territories.
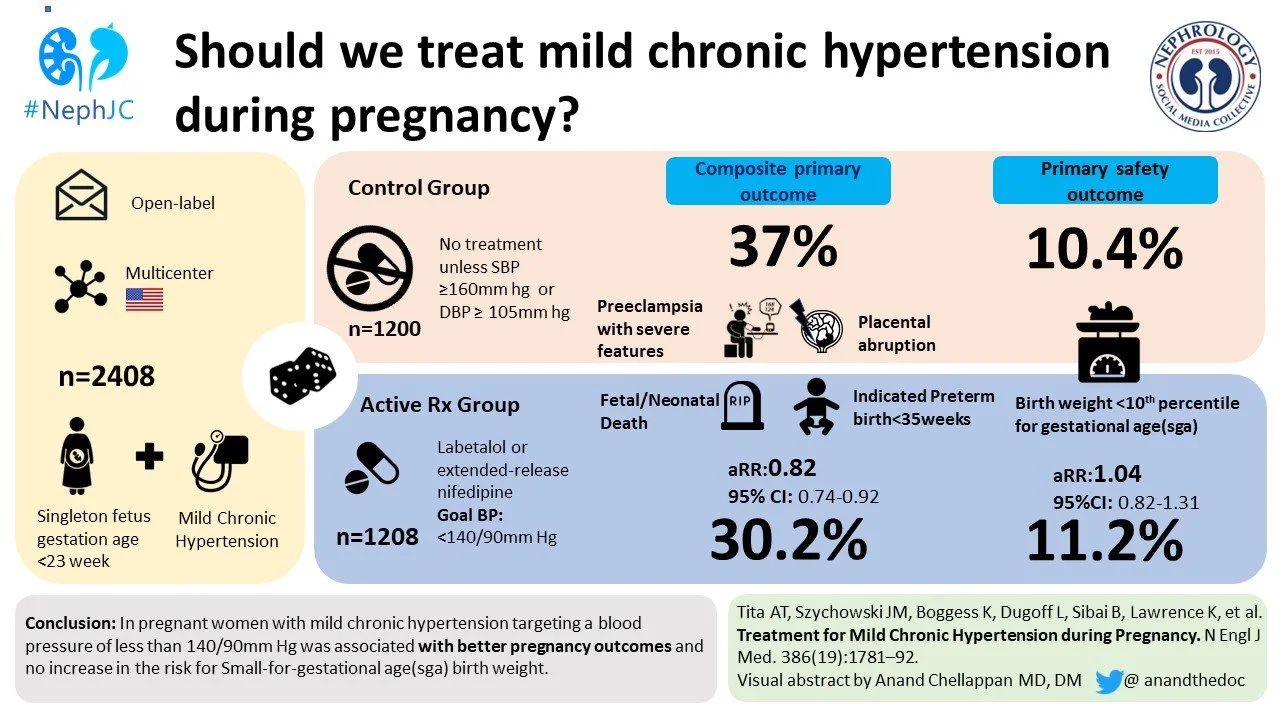
CHAP chips away at BP targets in pregnancy
The Chronic Hypertension and Pregnancy (CHAP) Trial addressed an important question – Is active treatment of mild-to-moderate hypertension in pregnancy safe for the fetus AND beneficial for the mother? Yes and yes. Pregnant patients in whom moderate hypertension was actively treated had lower incidence of primary outcome events including severe preeclampsia, premature birth, placental abruption or fetal or neonatal death. Over 2,400 patients were randomized to the active-treatment group (blood pressure goal <140/90 mmHg) or to the control group (antihypertensive medications stopped at randomization, only restarted if severe hypertension developed). A primary outcome event occurred in 30% patients in the active-treatment group, as opposed to 37% patients in the control group; adjusted risk ratio for a primary outcome was 0.82 (95% confidence interval 0.73 to 0.92, p < 0.001), with a number-needed-to-treat of 14.7. This trial was a follow-up to the 2015 study CHIPS that similarly showed no difference in risk of pregnancy loss or high-level neonatal care with tight-control vs less-tight of blood pressure during pregnancy. Overall CHAP was a large, well-designed trial that included an ethnically diverse population. While the results of the trial did not provide a specific target blood pressure for pregnant patients with chronic hypertension, it did provide further reassurance of safety and further benefit from starting antihypertensive therapy at a lower threshold of >140/90 mmHg (compared to previously recommended >165/110 mmHg). Promptly after publication of CHAP, the American College of Gynecology quickly updated their guidelines and released a practice advisory to its members. Now, if we could just get some alternative agents to use other than nifedipine, labetalol, and *shudders* hydralazine…
Dr Quaggin burns the barn down with her speech to kick off ASN Kidney Week
A leader’s job is to look into the future and see the organization, not as it is, but as it should be. Due to the political, financial and workload burdens of the last year, burnout in medicine is rampant and the physician’s voice and autonomy are being threatened. Further controversy swirled as Florida passed laws marginalizing the LGBTQ+ community prior to the ASN meeting. Dr. Susan Quaggin approached these challenges with ferocity and grace noting that, “We are going to Orlando, and we are bringing our values with us.” Campaigns like #SockItToKidneyDisease, #850challenge, and #4KidneyHealth encouraged comradery, advocacy, education and research while bringing greater visibility to the global impact of CKD. These campaigns highlighted the fact that 90% of the 37 million in the United States and 850 million worldwide are unaware they even have CKD diagnosis. In her opening address to the ASN in Orlando 2022, Dr. Quaggin was a firebrand for kidney disease advocacy. Embracing change, Dr. Quaggin started off with the notion that, “Nephrology as we know it is dying, and that is the best thing possible”. This is of course a nod to the many advancements and innovations over the last decade that are improving kidney care for our patients. She then stated that the penultimate goal was to put an end to kidney disease worldwide. Impossible you say? Nothing is impossible, there are only things that have not yet been accomplished. The objective is to keep moving forward, and build upon our successes. Dr. Quaggin noted that while we invest $960 for each cancer patient for research, we only spend $18 per CKD patient. Without advocacy and financial support, change will come slowly, if at all. It has been 50 years since congress approved universal support of dialysis treatments for ESKD. Dialysis is not the answer, and Dr. Quaggin encouraged each of us to make the fight for patients with kidney disease personal. Without knowledge there can be no creativity, and there can be no knowledge without emotion. Dr. Quaggin concluded by remarking that the future for nephrology looks bright, but great change requires great effort.