#NephJC Chat
Tuesday, January 6th, 2026, 9 pm Eastern on Twitter (X)
In 2025…did you feel it?! The monumental shift in the understanding of pathophysiology and the concomitant breakneck advancement of therapies. For decades, and essentially my entire nephrology career, we have been trying inadequately to fend off the relentless tide of chronic kidney disease. Treatments were aimed at slowing disease progression so that maybe patients would be lucky enough to die from another organ failing before they needed dialysis. Some diseases, particularly the various flavors of glomerulonephritis, had horrendous prognoses associated with rapid GFR loss and inevitable ESKD. There were always excuses: GNs were too rare to study, a national registry was too complex, and there was no money in GN treatments to encourage pharmaceutical companies to enter the space. We had no preventive treatments that focused on stopping the origins of disease. None.
This decade, we’ve made baby steps toward wearable dialysis technology and increasing the transplantable kidney pool (xenotransplantation), but “precision medicine” is definitely where the largest leaps were found in 2025. Once again, IgA Nephropathy led the way, creating a roadmap for future success in other etiologies of CKD, and outpacing KDIGO 2025 guidance before the ink was even dry. Let’s not forget that the most common cause of ESKD, diabetic nephropathy, is also seeing advancements toward halting disease progression. Diabetic nephropathy now has four independently active and additive pillars of GDMT, and one of those pillars might be the key to halting or even reversing progression (wait and see, fingers crossed). Yes, the future of nephrology is bright if we can embrace change and overcome the barriers that inevitably limit global use of game-changing breakthroughs. The future is navigating innovation IMPLEMENTATION, focusing on REMISSION through precision medicine, and potentially REGENERATION of kidney filtration. Hopefully, we will not let this momentum slip away. There is something about 2025 that is making me cautiously optimistic that we have finally started down a path that seemed untraversable just a decade before. If you are not excited for the next 5 years in nephrology therapeutics, then you just haven’t been paying attention.
Brian Rifkin, #NephJC co-chief editor
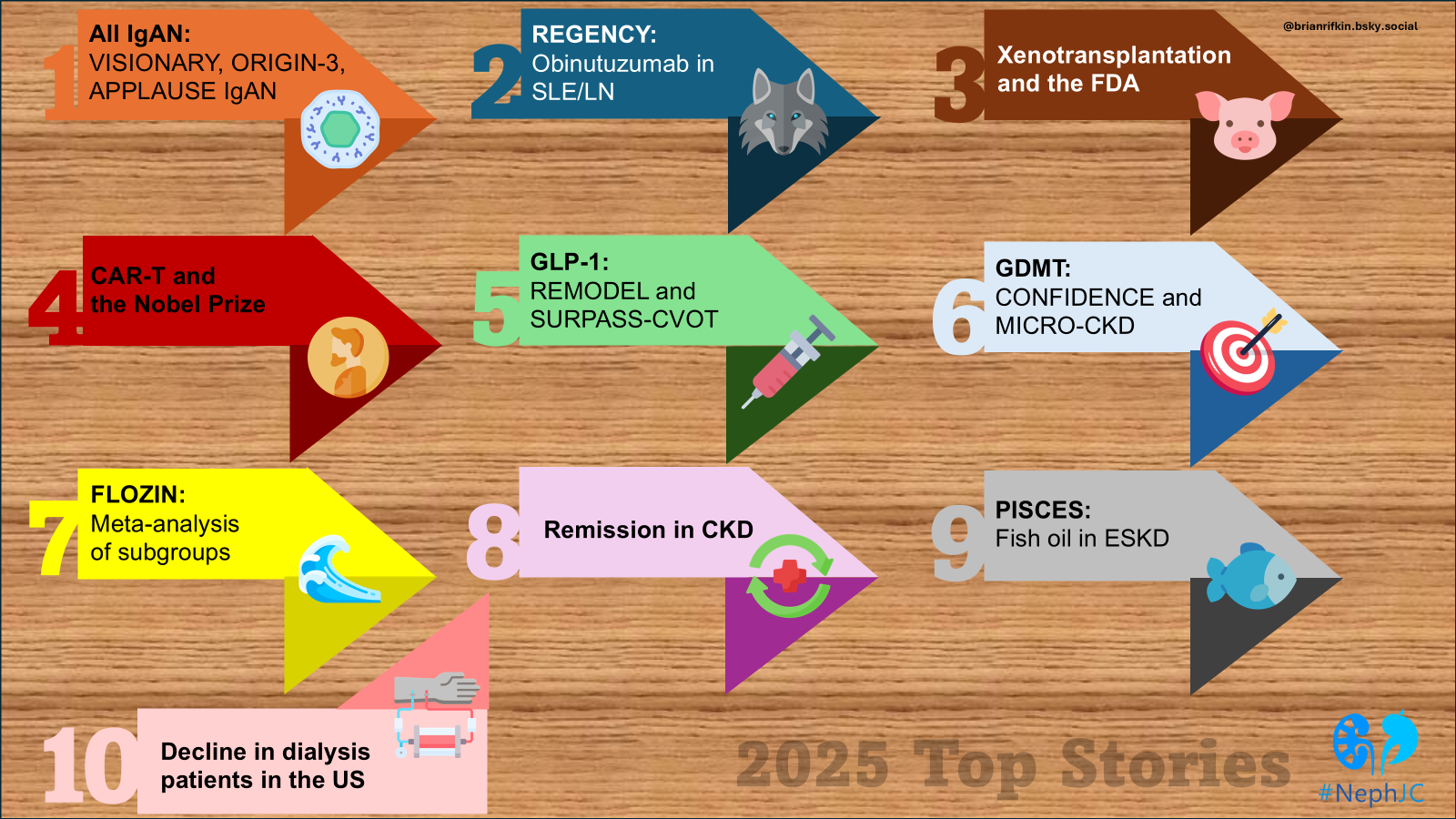
All IgA Nephropathy Trials: VISIONARY, ORIGIN-3, APPLAUSE IgAN
This year saw the “origin” of IgAN at the bull’s eye of innovation. With the addition of Nefecon and sparsentan to the age-old RASi and steroids, nephrologists were already enthusiastic in 2024. In 2025, new trials attacking the pathophysiologic origins of IgAN have left nephrologists with an embarrassment of riches (if only we understood how to incorporate them).
Phase 3 interim results of two new molecules were presented with simultaneous publications during Kidney Week. The ORIGIN-3 study (Lafayette et al, NEJM 2025|NephJC summary) is an ongoing multicenter, double blind, phase 3 trial of atacicept in IgAN. Atacicept is a human transmembrane activator and calcium-modulator and cyclophilin-ligand interactor (TACI)–Fc fusion protein, which inhibits two key immunoregulatory cytokines, BAFF and APRIL. Two-hundred and three patients (106 in the atacicept group and 97 in the placebo group) were randomised to receive either weekly subcutaneous atacicept or placebo. At 36 weeks, there was a marked reduction in the primary end point of 24-hour proteinuria in the treatment group vs placebo: 46% vs 7% (95% CI 28.9 -52.3; P<0.001).
Following close on its heels was VISIONARY (Perkovic et al, NEJM 2025), which studied the role of sibeprenlimab, a humanized monoclonal IgG2 antibody against APRIL. The trial enrolled 510 patients randomized to subcutaneous weekly sibeprenlimab versus placebo for 100 weeks. In the interim analysis, patients in the study arm showed a 51 % point difference in 24hr UPCR at 9 months versus placebo. The trial also demonstrated a decrease in pathogenetic molecules, including galactose-deficient IgA (by 67%) and APRIL (by 96%). Based on this trial, the FDA awarded accelerated approval to sibeprenlimab for IgAN. Both the ORIGIN-3 and VISIONARY trials are ongoing, and final results with endpoints assessing change in eGFR slope are eagerly anticipated.
The small molecule factor B complement inhibitor iptacopan had already proven its mettle in 2024 in the APPLAUSE trial by decreasing proteinuria in patients with IgAN by 38% when compared to placebo (Perkovic et al, N Engl J Med 2024). In October 2025, Novartis announced that the final results from the trial showed evidence of improvement in eGFR slope in patients on iptacopan, thereby strengthening its position in the IgAN treatment repertoire. The final data is expected to be released soon and to be used for the final FDA approval of the drug in 2026.
Last year also saw the accelerated FDA approval of atrasentan, a selective endothelin A receptor antagonist, based on the ALIGN trial, which demonstrated a 36% reduction in UPCR compared to placebo at 36 weeks (Heerspink et al, N Engl J Med 2024). The FDA also updated labeling for sparsentan, which had already received full approval for use in IgAN in 2024. With the updated labelling, based on review of safety data from PROTECT (Rovin et al, CJASN 2023|NephJC summary), DUPLEX (Rheault et al, NEJM 2023), and DUET (Trachtman et al, JASN 2018) studies, patients on sparsentan now need quarterly LFT monitoring after the first year, instead of monthly monitoring, which had earlier been advised.
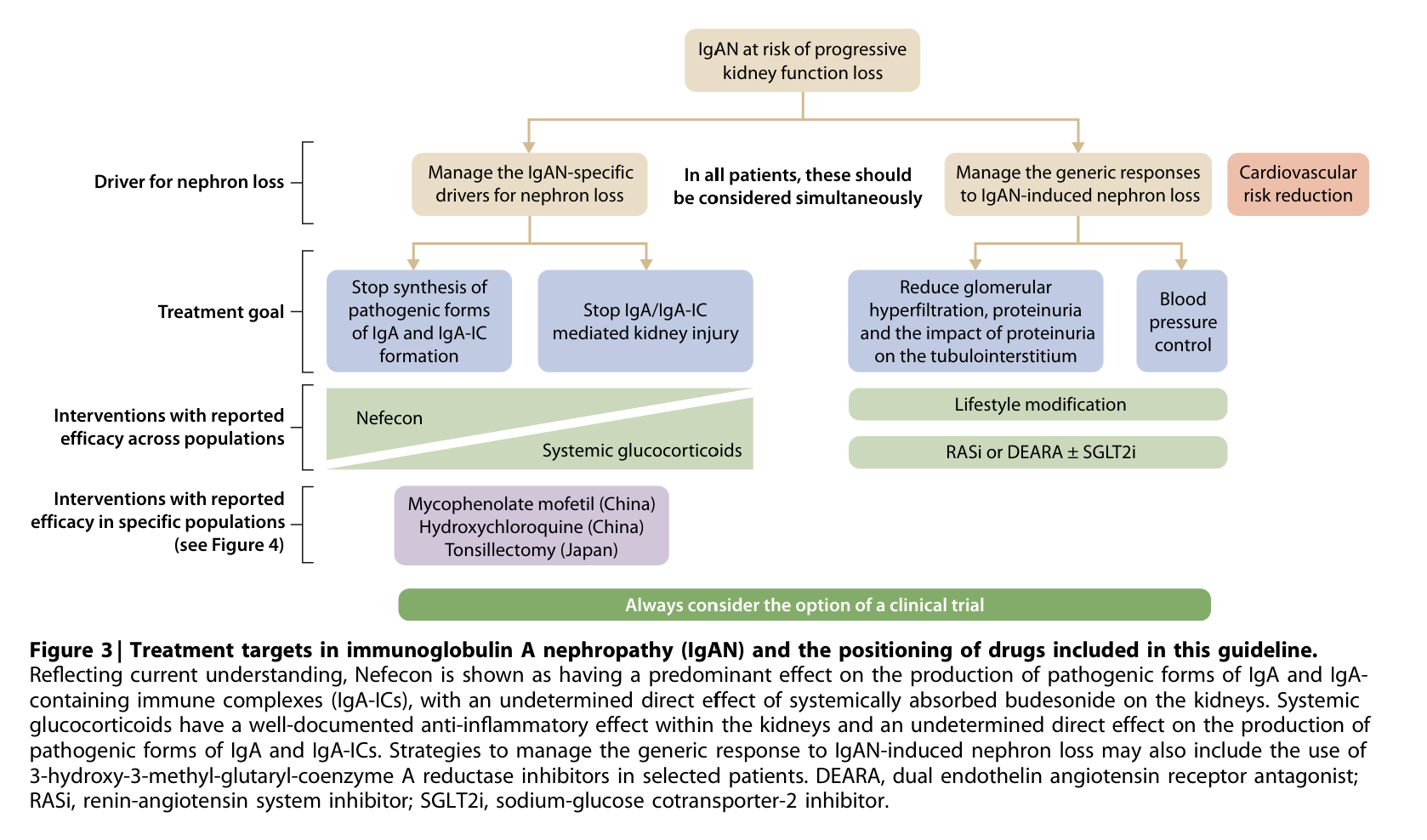
Recognising the advent of new molecules in this area, the KDIGO workgroup published the updated IgAN guideline in 2025 (NephJC summary). Based upon the risk of “progressive kidney function loss” in patients with IgAN with proteinuria >0.44 g/day from the RADAR cohort (Pitcher et al, CJASN 2023) the KDIGO guidelines (NephJC summary) now suggest a kidney biopsy in all patients with proteinuria >0.5 g/day and a suspicion of IgAN. The target proteinuria to prevent disease progression is suggested to be <0.5 g/day, and <0.3g/d where possible. Although the newly published trials have made many of the updates outdated, the current guidelines do suggest a paradigm shift in the way IgAN is managed. The guidelines suggested, as a practice point, to simultaneously target the two distinct pathways leading to kidney failure in IgAN: the IgAN-specific drivers and the generic drivers of nephron loss (figure 3).
Figure 3. Treatment targets in immunoglobulin A nephropathy (IgAN) and the positioning of drugs included in this guideline.- from KDIGO guideline IgA - 2025
There is even more in the pipeline to keep you gasping for air at the flood of therapeutics. Other molecules on the horizon showing promise in IgAN include teletacicept and povetaticept (both dual APRIL + BAFF inhibitors like atacicept; Madan et al, KIR 2026), zigakibart, a humanised IgG4 monoclonal antibody against APRIL (Kooienga et al, KI 2025), and mezagitamab, an anti-CD38 monoclonal antibody. This explosion of therapeutics is like drinking from a fire hydrant, and we still want more targeted treatments to assist in precision medicine. In which order should we implement these therapeutics and where do RASi and flozins fit in here? (listen to the podcast for some provocative discussion). However, implementation of newer treatments due to cost and logistics will remain challenging in the months and years ahead.
2. REGENCY: Obinutuzumab in lupus nephritis
By Tiff Caza
Systemic lupus erythematosus (SLE) impacts approximately 200,000 people in the United States, with 20-65% developing lupus nephritis (LN) at some time within their disease course. LN progresses to end-stage kidney disease (ESKD) in over one-third of cases and carries a 6-fold increased risk of mortality (Hocaoglu et al, Arthritis Rheumatol 2023). Only 40% of patients with LN achieve remission at 1 year despite immunosuppressive therapies, and therefore, new treatments (often as supplemental therapies) can be critical to obtain remission and reduce ESKD risk.
A kidney biopsy is required for a diagnosis of LN and is classified by the type of glomerular disease as defined by the International Society of Nephrology/Renal Pathology Society system (Bajema et al, Kidney Int 2018). These patterns of injury include minimal mesangial or mesangial proliferative LN (class I or II), proliferative LN (class III and class IV when active glomerular lesions are present in ≤50% or >50% of glomeruli, respectively), membranous LN (class V), and advanced sclerosing LN (class VI, representing ESKD). Proliferative LN comprises approximately 60% of cases, with an additional 12% of patients having a concurrent membranous component (class III/IV + class V). These patients have the highest frequency of progression to ESKD among LN classes (Hocaoglu et al, Arthritis Rheumatol 2023).
LN is an autoantibody-driven disease, and therefore, the use of B-cell depletion to treat LN has been examined. This was first explored with rituximab (a monoclonal antibody therapy targeting CD20+ B cells). While a promising strategy, LUNAR, a phase III placebo-controlled trial using rituximab as an add-on therapy, did not achieve its primary endpoints of a complete or partial renal response at 52 weeks (Rovin et al, Arthritis Rheum 2012). Incomplete B-cell depletion may have been a factor impacting the successful induction of remission. Long-lived plasma cells and memory B cells are not targeted by rituximab, which can allow for persistence of autoantibody production. Obinutuzumab is another candidate for B-cell depletion for LN, and is a type 2 anti-CD20 monoclonal antibody with enhanced binding that can induce antibody-dependent cell-mediated cytotoxicity, leading to more effective B-cell depletion.
The REGENCY trial sought to test the efficacy of obinutuzumab in LN. This was a phase III randomized, placebo-controlled clinical trial examining obinutuzumab as an add-on therapy in patients with proliferative LN with and without a concurrent membranous LN component (Furie et al, N Engl J Med 2025 | NephJC summary; Podcast). This study included 15 centers with 135 patients receiving obinutuzumab versus 136 receiving placebo. Both arms received standard of care therapy with mycophenolate mofetil and corticosteroids. The primary endpoint was a complete renal response at 76 weeks, defined as proteinuria ≤ 0.5 grams per day and eGFR ≥ 85% of baseline.
At 76 weeks, 46% of patients treated with obinutuzumab achieved a complete renal response, compared to 33% of patients in the placebo arm. A higher proportion of patients had significant reductions in proteinuria, with 56% of patients on obinutuzumab having ≤0.8 grams of proteinuria compared to 42% in the placebo arm. Another benefit was steroid-sparing effects, with a reduced steroid requirement in obinutuzumab-treated patients (43% vs. 31% requiring ≤ 7.5 mg/day). This is a similar rate of remission as achieved by treatment with belimumab, phase III BLISS-LN trial (Furie et al, N Engl J Med, 2020) and voclosporin phase III AURORA trial (Saxena et al, Arthritis Rheumatol 2024), which are now integrated in the KDIGO LN guidelines treatment algorithms.
Efficacy was seen in both patients with proliferative LN alone, as well as those with a concurrent membranous (MLN) component. In fact, patients with a MLN component or >3 grams of proteinuria/day were more likely to achieve remission compared to patients with proliferative disease alone. This may not be surprising as B-cell depletion is efficacious in membranous nephropathy with rituximab use as the standard-of-care (according to the KDIGO 2021 glomerular disease guidelines). Obinutuzumab is currently being evaluated within a phase III randomized controlled trial for efficacy in membranous nephropathy, seeking to improve relapse rates through more effective B-cell depletion (MAJESTY trial, clinicaltrials.gov NCT04629248).
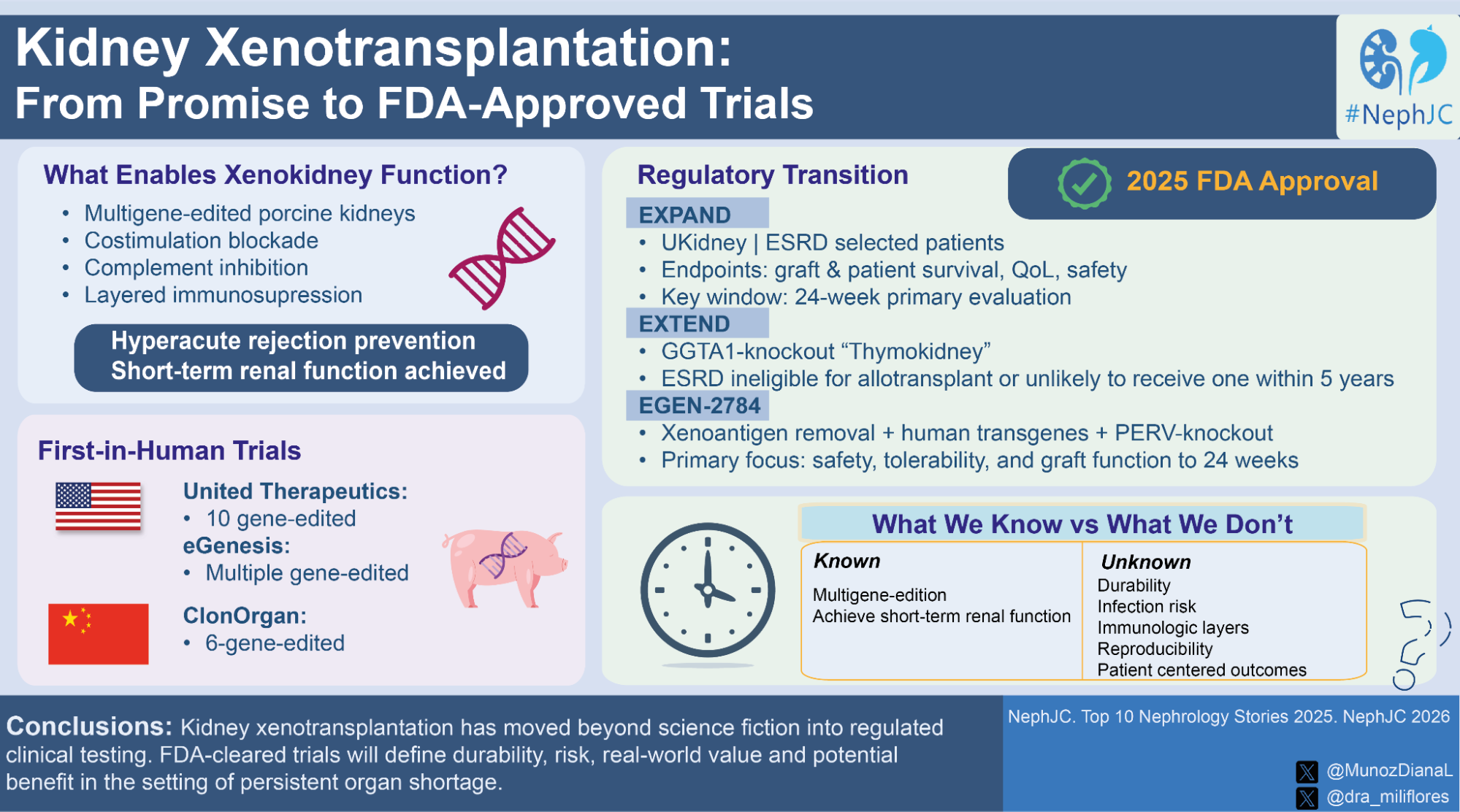
3. Xenotransplantation progress and the FDA approves further trials
As Swap noted again in the Top Stories of 2024, xenotransplantation returned to the Top 10 and is likely to stay for some time, as a rapidly advancing realistic RRT alternative. Far from a passing trend, xenotransplantation has entered a new developmental phase, moving beyond theoretical promise and experiments in brain-dead recipients toward the first trials in living humans, a genuine inflection point for the field.
Early clinical translation has shown that multigene-edited porcine kidneys, when combined with contemporary immunosuppressive strategies, can overcome hyperacute rejection and achieve sustained renal function for weeks to months. While these findings represent a major biological advance, they come from highly selected cases and evolving protocols, with variable outcomes and evidence largely from case reports (Tao et al, Front Immunol 2025). Illustrative examples include Towana Looney, who maintained xenokidney function for 130 days before developing acute rejection and Tim Andrews, whose graft persisted for 271 days before being removed due to persistent proteinuria and declining eGFR. Together, these cases highlight both the excitement and the unresolved risks of clinical xenotransplantation.
That’s exactly why 2025 marks the transition into formal, FDA authorization. The FDA granted IND clearance for United Therapeutics to launch the EXPAND trial (NCT06878560), a prospective study of a 10-gene edited xenokidney in selected ESRD patients, with a 24-week evaluation window focused on patient and graft survival, quality of life, and long-term follow-up (Eisenson et al, Nat Commun 2024). In parallel, United Therapeutics registered EXTEND (NCT07224763) to explore the safety and efficacy of GGTA1-knockout (Thymokidney), targeting ESRD patients who are either ineligible for conventional allogeneic kidney transplant or unlikely to receive a transplant within 5 years despite waiting list.
In contrast, eGenesis has received IND clearance for EGEN-2784, a phase 1/2/3 program based on a more extensive multiplex gene-edited porcine kidney from Yucatan miniature pigs. These researchers incorporated elimination of major glycan antigens, insertion of multiple human immunoregulatory and anticoagulant genes, and complete inactivation of porcine endogenous retroviruses (PERVs), reflecting a design philosophy aimed at maximizing long-term immunologic compatibility and infectious safety (Kawai et al, N Engl J Med 2025). Beyond the United States, ClonOrgan Biotechnology has reported living-human kidney xenotransplantation in China, where a 69-year-old woman received a 6-gene–edited porcine kidney 6-gene–edited porcine kidney from Bama miniature pigs and has demonstrated ongoing graft function approaching eight months (Peng et al, Biotechnol J 2025). However, these procedures have not yet been conducted within registered and protocolized clinical trials, limiting direct comparison.
Taken together, kidney xenotransplantation is no longer science fiction, but it is not yet “primetime” either, as significant challenges remain. The true contribution of the next generation of trials will be less about proving that xenokidneys can work, but more about defining what is their value and risk, compared with existing therapies. Can further modifications decrease rejection risk and improve organ longevity? When it becomes clinically available, what will be its cost? Until those data emerge, xenotransplantation should be viewed as a rigorously tested experimental strategy, with rapidly evolving innovation. Unfortunately, in its fourth appearance in Top Stories, it remains only the forerunner of a solution to the persistent kidney organ shortage and urgent need to remove patients from dialysis.
4. Nobel prize winner and peripheral immune tolerance: kidney transplantation application and CAR-T in nephrology
The 2025 Nobel recognition of immune tolerance biology and T-regulatory circuitry set a scientific foundation for interventions capable of restructuring, rather than suppressing, immunity. Autoimmune kidney disease and kidney transplantation both hinge on the same failure: unstable, self-directed, or allo-directed lymphocyte networks that cannot be durably silenced by conventional immunosuppression.
CAR-T (Chimeric Antigen Receptor T-cell) platforms operationalize this new immunology. CD19 CAR-T consistently clears autoreactive B cell pools in refractory SLE and lupus nephritis, with full B- and plasmablasts depletion confirmed in both blood and marrow early trials and case series. (Mackensen A et al, Nat Med. 2023| NephJC summary) However, CD19 alone does not reach long-lived plasma cells, which continue to produce pathogenic antibodies.
Dual-target CAR-T strategies extend depletion to BCMA-expressing (B-cell maturation antigen) long-lived plasma cells. A phase 1 study using CD19+BCMA CAR-T mapped autoreactive clones to marrow plasma cells and showed these cells persist after single-target therapy but are eliminated with dual targeting (Feng J et al. Nat Med. 2025). Clinically, most patients experienced marked reductions in proteinuria, normalization of complement, and broad autoantibody clearance. Immune profiling showed suppression of interferon-stimulated gene activity in both blood and marrow, along with contraction of BAFF and FLT3 signalling- key pathways supporting autoreactive B-cell survival. B-cell reconstitution was dominated by IgM/IgD naive cells, indicating removal of mature class-switched autoreactive clones rather than temporary suppression. This pattern is consistent with an immune reset.
These same mechanisms point toward future curative intent in other immune kidney diseases. Autoantibody-driven pathologies- ANCA vasculitis, membranous nephropathy with refractory PLA2R (Yang Y et al. JASN, 2025), and MGRS with nephrotoxic clones (Al Haddad N et al. JASN, 2025) - share dependence on B-cell maturation networks and plasma cell persistence. CAR-T achieves deeper and more durable depletion than monoclonal antibodies, bispecific engagers, or small-molecule immunosuppressants, which leave long-lived plasma cells intact and require chronic exposure. (Tondolo F et al, Ren Fail, 2025).
Transplantation provides another frontier. Alloimmunity is an engineered autoimmune state. Highly sensitized candidates, unable to clear memory anti-HLA clones even with aggressive desensitization, represent a logical target. (Jarmi T et al, Kidney Int, 2024) A mechanistic precedent exists: early biopsy data show CAR-T infiltration into secondary lymphoid tissues and depletion of germinal-center-like B-cell aggregates within inflamed organs, suggesting an ability to extinguish alloantibody production at its root (Tur C et al. Ann Rheum Dis. 2025). This aligns with emerging work on donor-recipient immune chimerism, where durable tolerance correlates with coordinated recalibration of T-reg networks and extinguishing of memory alloantibody responses. CAR-T mediated removal or resistant B-cell lineages creates biological conditions similar to mixed chimerism protocols but without marrow-toxic conditioning. (NephJC commentary)
The logic is straightforward. The kidney is often the first organ where autoimmune damage becomes clinically irreversible, and the last organ where chronic immunosuppression leaves an acceptable margin of safety. A therapy capable of resetting the underlying lymphocyte architecture- the axis highlighted by the 2025 Nobel work on regulatory tolerance- opens a path to cure versus merely control. Among available technologies, CAR-T and its descendants (dual-target constructs, chimeric auto-antibody receptor T cells, bispecific T-cells redirectors) are the first to demonstrate mechanistic and clinical plausibility for achieving that goal through the whole immune system rather than isolated cytokine pathways. (NephMadness 2025: CAR-T for Kidney Disease Region)
5. GLP-1 RA progress: REMODEL, SURPASS-CVOT
By Joel Topf
When the history of GLP-1 RAs in kidney disease is finally written, 2024 will always be the highlight, because of the central importance of the FLOW trial (Number 1 on last year’s Top Stories). However, 2025 added important evidence to the validity of the FLOW trial and let’s face it, drugs this powerful are going to make the Top Stories list for years to come (2 and counting) as newer “G-force” molecules and understanding of physiological mechanisms improve.
If FLOW defined the “WHAT” semaglutide offers patients with diabetic nephropathy, REMODEL is attempting to define the “HOW” semaglutide provides that nephroprotection. REMODEL brings advanced scientific methods to look at what is going on inside the kidney with GLP1-RAs to provide nephroprotection. Here is how the investigators describe the trial.
“REMODEL is a 52-week, multinational, randomized, double-blind, placebo-controlled MoA trial comparing once-weekly s.c. semaglutide (1.0 mg) with placebo in people with CKD and T2D. Participants in REMODEL underwent advanced functional MRI (fMRI), including blood oxygenation level-dependent (BOLD) MRI, phase-contrast MRI, and T1 mapping, and examination of blood and urine biomarkers at baseline and after 4 and 52 weeks of treatment. A subgroup had paired kidney biopsies with morphologic and molecular analyses, which included single-nucleus RNA sequencing (snRNAseq) and spatial transcriptomic”
- Pruijm M et al, Kidney International 2025
This was announced at Kidney Week 2025, and we can expect exciting publications to come from this unique trial design to come out during 2026 and beyond. The dream is that unlocking the secrets of nephroprotection in diabetic kidney disease will allow newer, less toxic, and more effective therapies to blossom.
Additional outcomes data supporting the results of FLOW were released in 2025. The highlight was the renal results of the SURPASS-CVOT trial, a head-to-head noninferiority cardiovascular outcomes study comparing tirzepatide to dulaglutide. The primary analysis (Nichols et al, NEJM 2025) examined MACE and showed that tirzepatide was non-inferior to dulaglutide. The kidney outcomes, however, were quite a bit more impressive. From the abstract:
“Changes in eGFR at 36 months were −3.0 (0.5) for TZP and −7.2 (0.4) for DULA with a significant difference of 4.1 mL/min per 1.73 m2. The percent changes in UACR at 36 months were −45.6 for TZP and −28.0 for DULA with a significant difference of -24.6 g/kg. The risk of the four-component composite kidney outcome was 33% lower with TZP treatment than DULA treatment (108 [16.7%] versus 137 [23.0%], HR 0.67, 95% CI 0.52 to 0.87, p=0.002).”
- Zoungas et al, JASN 2025
The GFR results are stunning; the tirzepatide group lost 3 ml/min over three years, which is equivalent to age-related (background) loss of GFR. Utterly remarkable. The other important aspect of SURPASS-CVOT is that it is a trial with active controls. We live in a post-FLOW world, and we should not let our patients be randomized to placebos in GLP-1 RA trials.
In other important 2025 GLP-1 RA news, semaglutide got a formal indication for reducing the risk of GFR decline, kidney failure, and death from cardiovascular disease in adults with type 2 diabetes and chronic kidney disease. The cardio-kidney-metabolic disorder now has a GDMT therapy that is highly effective and disrupts the interorgan decline associated with patient morbidity and mortality.
Taken together, 2025 moved GLP-1–based therapy in nephrology from proof to precision—using REMODEL to interrogate mechanism and SURPASS-CVOT to show that, even against active comparators, incretin biology can nearly normalize kidney function trajectories in high-risk patients.
6. GDMT implementation in CKD: lessons learnt from CONFIDENCE and MIRO-CKD
Guideline-directed medical therapy (GDMT) has transformed outcomes in heart failure and cardiorenal disease, yet patients with chronic kidney disease continue to receive it less often and at much lower doses than those without CKD. This is not because the evidence is weak. RAS inhibitors (RASi), non-steroidal mineralocorticoid receptor antagonists (nsMRAs), and SGLT2 inhibitors (SGLT2i) have each shown clear reductions in CV and kidney outcomes across multiple landmark trials. Yet in everyday practice, CKD often triggers hesitation (or even retreat from GDMT), rather than urgency. Many clinicians fear hyperkalemia, hypotension, and worsening eGFRs, despite the presence of robust efficacy data. Over time, this very fear translates into therapeutic inertia. CONFIDENCE (Agarwal et al, NEJM 2025 |NephJC summary; podcast) and MIRO-CKD (Heerspink et al, Lancet 2025) enter this space not as efficacy trials, but as implementation trials, asking a more practical question: Can we accelerate and maintain GDMT initiation in CKD?
The CONFIDENCE trial attempted to directly test this idea. The trial asked whether combination therapy (nsMRA and SGLT2i) can be started simultaneously, rather than sequentially over months. This multi-centre, randomized, double-blind study compared simultaneous finerenone plus empagliflozin against each agent alone on a background of RAS blockade. The trial enrolled 818 diabetics with CKD (eGFR ~30–90 mL/min/1.73 m² and UACR ≥100 to <5000 mg/g, HbA1c <11%, and were on maximally tolerated RASi for >1 month), who were randomized 1:1:1 to finerenone plus empagliflozin, finerenone alone, or empagliflozin alone, with dosing stratified by baseline eGFR and UACR. Patients were followed for UACR change and a composite kidney outcome. By 6 months, the combination therapy achieved a 52% reduction in UACR, which was 29% greater than finerenone alone and 32% greater than empagliflozin alone, with most of the effect seen within 4 weeks. The combination was safe, with low rates of symptomatic hypotension and AKI. The safety data emphasized modest increases in hyperkalemia incidence but no large excess in discontinuations when monitored per protocol. We will need to continue to educate our primary care physicians about the need to restart and maintain GDMT, especially after hospitalization for acute illness.
MIRO-CKD complements this by focusing on one of the most anxiety-provoking areas of GDMT in CKD: mineralocorticoid receptor modulation. MIRO-CKD was a multicentre, double-blind, active-controlled phase 2b dose-finding study that took 324 participants with eGFR 25 to ≤60 mL/min/1.73 m², UACR >100 to ≤5000 mg/g, and serum potassium 3.5–5.0 mmol/L, on background RAASi. Patients were randomised 1:1:1 to dapagliflozin 10mg + either balcinrenone 15 mg / balcinrenone 40 mg OR placebo for 12 weeks. Among the participants (mean eGFR ~42 mL/min/1.73 m²; median UACR 365 mg/g), both balcinrenone doses combined with Dapagliflozin significantly reduced albuminuria compared with dapagliflozin alone. At 12 weeks, UACR was lower by 23% with balcinrenone 15 mg and by 33% with balcinrenone 40 mg versus dapagliflozin alone, showing a clear dose–response. Hyperkalemia rates were low and similar across groups (6–7% with balcinrenone vs 5% with dapagliflozin alone), hypotension and renal adverse events were infrequent, and no unexpected safety signals emerged. With prespecified monitoring and dose adjustments, severe hyperkalemia and discontinuation were uncommon- data that tell us how to use nsMRAs in CKD rather than simply asserting they are safe in principle. Still, the question remains, will this be consistent in a non-clinical trial setting?
Risk-stratification with frequent lab checks, use of titration algorithms (temporary hold thresholds, modest down-titration steps, and use of potassium binders when indicated), may help clinicians accelerate implementation. These trials don’t argue for recklessness; they argue for systematized courage: treat the underlying risk, anticipate the predictable derangements, and use the tools (protocols, binders, close follow-up) to keep CKD patients on life-prolonging GDMT therapies.
7. FLOZIN meta-analysis across all subgroups (2X SMART-C and EMPA individual level meta-analysis)
By Marc Soco
What began as glucose-lowering therapy for type 2 diabetes has quietly, and then decisively, rewritten the rules of cardiorenal medicine. Along the way, the nephrology community found a rallying cry: “flozination”, capturing not merely a drug class but a conceptual shift. It reflected a collective realization that this was more than another incremental guideline update. Early on, SGLT2 inhibitor use was framed cautiously: type 2 diabetes, significant albuminuria, and preserved eGFR. However, these constraints mirrored trial inclusion criteria rather than biological plausibility; but nephrologists are, by necessity, skeptics. We waited for kidney outcomes – and they arrived. First as secondary endpoints, then as trials explicitly designed for kidney disease: CREDENCE (Perkovic et al, NEJM 2019 | NephJC Summary), DAPA-CKD (Heerspink et al, NEJM 2020 | NephJC Summary), and EMPA-KIDNEY (Herrington et al, NEJM 2022 | NephJC Summary), each progressively widening eligibility. As repeatedly emphasized in NephJC discussions, the signal remained consistent even as study populations broadened (should we just add them to the water supply?)
Flozination became shorthand for a mindset: trust outcomes over labels; follow risk rather than rigid categories; and treat the heart and kidney as inseparable organs. What individual trials suggested, meta-analyses have now confirmed. In 2022, we had an early preview of where the evidence was heading. Across large placebo-controlled trials, benefits were consistent in people with and without diabetes (Baigent et al, Lancet 2022). This served as a critical bridge, helping the field move beyond a diabetes-centric framing of SGLT2 inhibitors. It set the stage for a fully cardiorenal view, where baseline risk and not diagnostic labels determine benefit.
The SMART-C (SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium) (Neuen et al, JAMA 2025) is not a single trial but a collaborative meta-analysis pooling data from nearly all large, randomized, placebo-controlled SGLT2 inhibitor outcome trials. This approach enabled investigators to address questions no single study could definitively answer: Do benefits depend on diabetes status? On albuminuria? On baseline eGFR? Across tens of thousands of participants, the answer was consistently no, patients benefited universally.
Neuen et al’s meta-analysis demonstrates that the benefit of SGLT2 inhibitors does not diminish with declining kidney function. Relative reductions in kidney disease progression were strikingly consistent across the full eGFR spectrum, from preserved function down to <30 mL/min/1.73 m², despite fewer functioning nephrons. This directly challenges the long-held assumption that therapies must lose efficacy as filtration falters and reframes low eGFR not as a limitation, but as a marker of higher absolute opportunity for benefit.
In contrast, Staplin et al confirms that diabetes and albuminuria modify absolute risk, not relative treatment effect. While higher albuminuria and diabetes amplify event rates, the proportional reductions in kidney and cardiovascular outcomes remain consistent across strata. In other words, albuminuria and diabetes identify who stands to benefit most, not who stands to gain at all (Staplin et al, JAMA 2025).
Taken together, these findings show that the slope does not flatten as filtration declines and that the signal does not depend on metabolic labels. What remains is a unified message: flozins track cardiorenal risk, not diagnostic categories.
If the SMART-C collaborative meta-analysis confirmed cross-category benefit, the EMPA individual participant-level meta-analysis delivered depth. This individual participant-level meta-analysis pooled raw patient-level data from four large, placebo-controlled empagliflozin trials: EMPA-REG OUTCOME (Zinman et al, NEJM 2015), EMPEROR-Reduced (Packer et al, NEJM 2020), EMPEROR-Preserved (Anker et al, NEJM 2021), and EMPA-KIDNEY. It encompassed more than 23,000 participants. Compared with placebo, empagliflozin reduced the risk of AKI markers by ~20%, AKI adverse events by ~27%, CKD progression by ~30%, and kidney failure by ~34%, with statistically consistent effects across subgroups defined by predicted acute eGFR dip and other clinical characteristics. These individual-level data reinforce that relative benefits are preserved across the cardiorenal risk continuum (Herrington et al, Lancet 2025 | NephJC Summary). Collectively, the evidence favors risk-guided therapy over traditional indication-based prescribing.
Flozins have become foundational cardiorenal therapy, not because guidelines have fully caught up, but because outcomes demand it. Beyond improving endpoints, flozins have reshaped how nephrologists think. They have moved the specialty firmly into preventive medicine, strengthened collaboration with cardiology, and validated years of FOAMed discussion, NephJC debates, and social-media scholarship that argued that we were underusing a transformative therapy.
Flozination was never hype. It was pattern recognition. And now, with these meta-analyses, the evidence has caught up with the intuition.
8. Paradigm Shift: Aiming for CKD Remission
By Elba Medina & Cristina Popa
For decades, nephrology defined success defensively: slow the slope, delay dialysis, accept decline in eGFR as predetermined fate. The paradigm has made a marked change in 2025, and now we nephrologists are on offense. CKD is no longer intrinsically progressive. CKD remission (eGFR decline of < 1 mL/min/1.73 m² per year) has become a credible, practical, and increasingly achievable therapeutic goal. Earlier frameworks already hinted at this possibility. Observational data showed that a substantial proportion of patients experienced regression or even normalization of kidney function, particularly when albuminuria was low, and blood pressure was controlled (Ruggenenti P et al, Lancet, 2001| Taal MW, Curr Opin Nephrol Hypertens, 2022). Yet, these signals were marginalized, partly because nephrology lacked a language for eGFR improvement. Regression and remission were poorly defined, methodologically inconvenient, and philosophically discordant with a field built around inevitable, irreversible eGFR loss.
The last decade forced a recalibration in nephrology. During Kidney Week, when Vlado Perkovic presented this astounding perspective, it echoed in the hearts (and kidneys) of many; we no longer had to just settle for unavoidable ESRD for our patients (Tangri N et al, Kidney Int, 2025). Combination therapy with RAAS blockade, SGLT2i, non-steroidal MRAs, GLP1-RAs, and disease-specific immunotherapies has now reliably and repeatedly altered eGFR trajectories. eGFR slopes (once measured at -5 to -10 ml/min/1.73m2 per year) now approach what many consider in line with physiological aging eGFR decline. Albuminuria reductions exceeding 60-80% are no longer exceptional. In both IgAN and DKD, sustained eGFR preservation and biochemical normalization are reproducible outcomes across many disease phenotypes.
The shift is more than semantic; it reframes CKD from an exercise in damage control to one of organ preservation. Remission, defined pragmatically by stable eGFR slopes below 1 ml/min/1.73m2 per year or normalized eGFR, or normo-albuminuria (< 30 mg/day)- demands earlier detection, aggressive combination therapies, and intolerance to therapeutic inertia. It also demands clinical trials, updated guidance, and a healthcare system that finally abandons CKD progression as the sole endpoint of relevance.
Nephrology has crossed a conceptual threshold. But can we dare to speak of curing CKD? Even if we can’t say yes quite yet, the question is no longer naive, as illustrated in this National Geographic perspective. Perhaps xenotransplantation won’t be the answer to RRT if we can determine the signals to nephron regeneration in the near future.
9. Fish Oil in Dialysis: PISCES
Once upon a time, Lord Vishnu saved our sacred scriptures (epics and vedas) from asuras (demons) in mathsya (fish) avatar. Drawing the same parallel, will the same fish come to rescue the patients on maintenance hemodialysis who have a higher risk of mortality due to cardiovascular events?
Many studies have been published about the antioxidant and anti-inflammatory properties of fish oil, as it is rich in polyunsaturated fatty acids (PUFA). The two main ones that have received attention include Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA). It is strongly held that the proportion of these two molecules is critical to their interaction with cardiac cell membranes, and their subsequent clinical benefits to arrhythmia and sudden cardiac death (a major mortality outcome in hemodialysis patients). Fish oil has been tried in many clinical trials to prevent cardiovascular events in the general population with variable results. This is not surprising given the complex heterogeneity of various fish oil supplements and the lack of a pathophysiologic mechanistic understanding (we don’t know which parts are critical, and in what form or amount).
Hemodialysis patients seem to be an easily identified population to study the benefits of fish oil: 50% of all deaths in hemodialysis are due to CV events (10-20 times higher than the general population). This is because of the combined effect of both traditional risk factors (diabetes, hypertension, obesity) and non-traditional risk factors (chronic inflammation, oxidative stress, endothelial dysfunction, disordered mineral metabolism, sympathetic activation, intradialytic electrolyte shifts). There has been research done to attempt to reduce the CV mortality rate in dialysis patients, but most of the studies failed to show a benefit. Perhaps the most impressive thus far has been the use of hemodiafiltration (HDF), but universal acceptance of this modality, and its benefits to hemodialysis patients, is still lacking (Rose et al, Kidney Int 2024).
PISCES (protection against incidences of serious CV events study) (Lok CE, et al, NEJM, 2025 | NephJC summary; podcast) was a well-designed trial. Initially started as a pilot trial, later extended to a proper clinical trial as a multi-center, double-blinded RCT across 26 dialysis centers in two countries (Canada and Australia). 1300 patients were randomized to fish oil (4gm, with 0.8 grams DHA and 1.6 grams EPA) group versus corn oil (placebo). The primary endpoint, fatal and non-fatal cardiovascular events, was lower in the fish oil group with an HR of 0.53! The same fish oil has had no significant benefits in the general population (HR 0.99) in previous studies. It has been shown previously that hemodialysis patients are deficient in DHA/EPA, and thus replacement to near normal levels might have specific advantages in these patients (Khor et al, Nutrients 2018). Even the extended primary outcomes (secondary outcomes), non-cardiac death (HR 0.77), cardiac death (HR of 0.55), peripheral vascular disease leading to amputation (HR 0.57), fatal and nonfatal myocardial infarction (HR 0.56), fatal and nonfatal stroke (HR 0.37) were lower in the fish oil group. Even the safety assessed by bleeding events was lower with the fish oil group. With such a huge magnitude of effect/benefit, many a skeptic was left scratching their heads.
Table 2. Primary and secondary endpoints, from Lok CE, et al, NEJM, 2025
Could fish oil be the panacea we’ve been looking for (or just another fish-in-the-sea-uh)? It will be hard to sit on this knowledge and wait for confirmatory studies, given the low cost and high potential benefit. More than one nephrologist, when confronted with the issue of “high pill burden” and compliance, has suggested ditching the phosphorus binders (which lack any mortality benefit). Finally, in terms of implementation, if we believe that it is this specific formulation (with its quality and ratio of DHA/EPA that is the secret sauce) that benefits hemodialysis patients, then availability has definitely been an issue. The company that manufactured and donated the fish oil for the study has subsequently been sold twice. Fear not, given the publicity and interest, the study authors are working on increasing the availability of this specific formulation on a larger scale. Until then…go fish.
10. Decline in Dialysis Patients in the United States
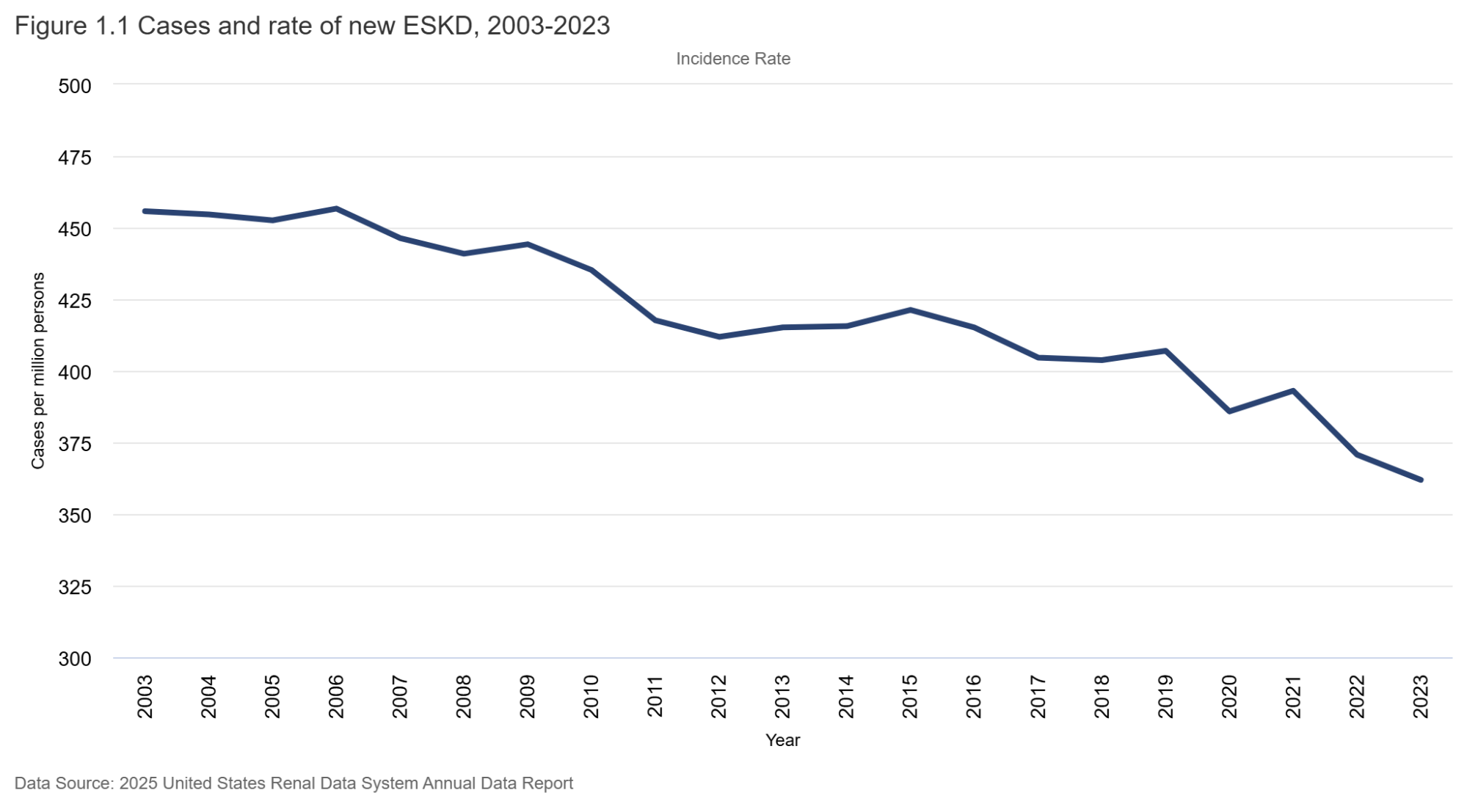
CKD is an epidemic, with about one in 7 individuals having CKD, an estimated 788 million worldwide. In 2023, about 1.5 million adults died of CKD-related causes worldwide. Dubiously, “kidney failure” is inching up as a cause of death in the morbidity rankings, number 9 globally, just behind diabetes but ahead of lung cancer. (All stats from the Global Burden of Disease study, Lancet 2025, see NephJC summary). But has this relentless, dire stream of news masked an underlying optimistic trend? One of the best long-term sources of information (at least since 1988) has been the United States Renal Data System (USRDS) Annual Data Report (ADR), which contains updated information about the CKD and ESRD populations in the U.S. (see NephJC coverage from 2023 and see this talk from Eric Weinhandl before reading further). In this story we will cover one small aspect of the 2025 USRDS report: the change in incidence of ESRD over time.
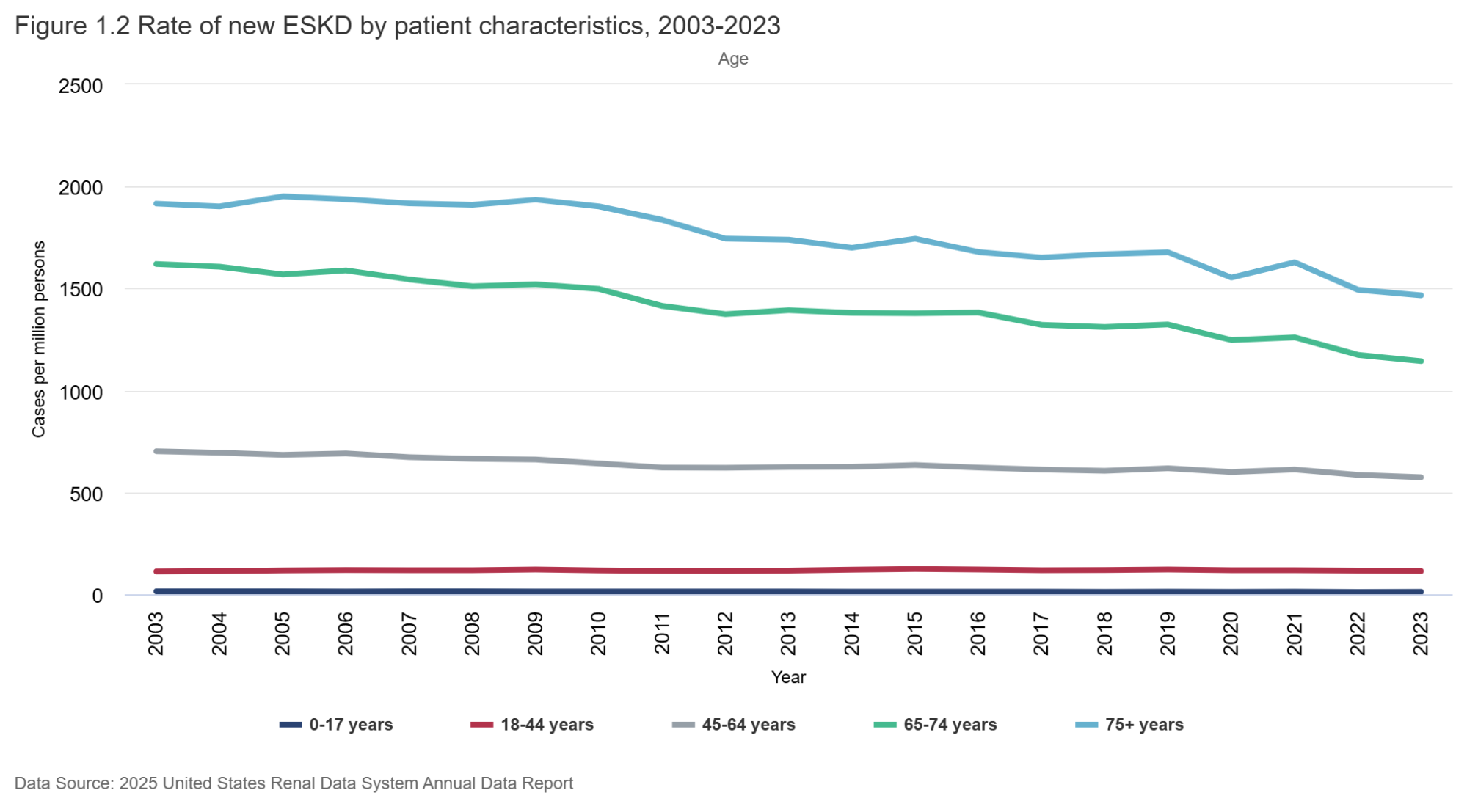
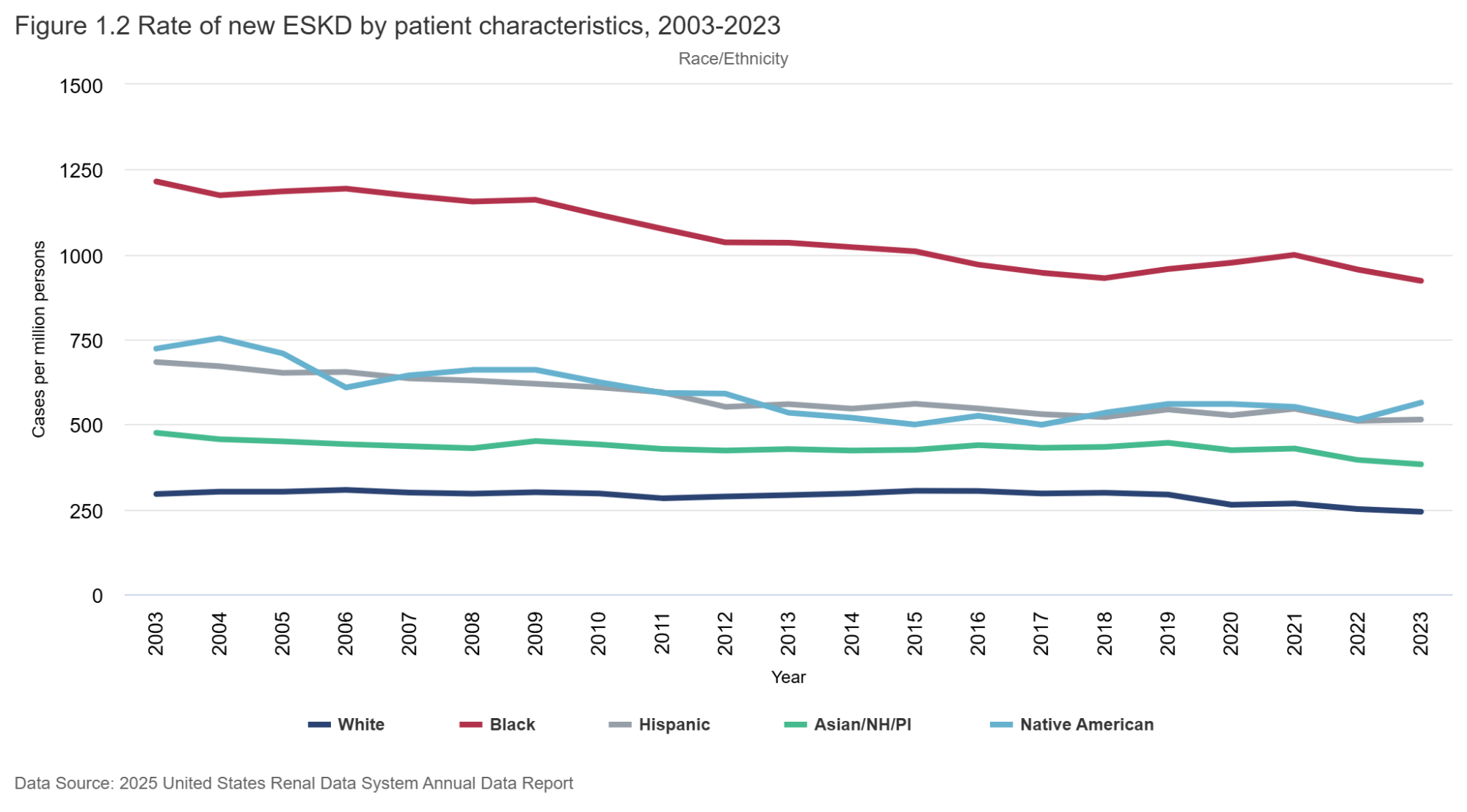
Figure 1.1 (below) shows how the incidence rate has changed over time. From a peak of about 460 ESRD cases per million population in 2006, the rate has steadily dropped - down to a low of 362 per million in 2023. That’s an astounding reduction of about 20%, or 100 per million population in the last 17 years. It is seen in men and women - and in all race/ethnicity groups (with some small blips upwards in Black/Hispanic groups). Why might this be so? As far as the rate of decline goes, we can ascribe this to many things: RENAAL (Brenner et al, NEJM 2001) was published in 2001; CKD staging was pushed out in 2002 (Levey et al, Annals of IM 2002), which seems like two important preceding events of consequence. As one would expect, it would take a few years of RAS inhibition and awareness of early-stage CKD to influence “better care” and manifest as declining CKD incidence. Possibly the IDEAL trial (Cooper et al, NEJM 2010) may have also contributed in the 2010s to later (and lower) dialysis starts, as early initiation of dialysis therapy failed to show any clinical or survival benefits. Certainly, we can hope that flozination and G-force (SGLT2i and GLP1-RA) implementation will keep driving these rates lower as time goes on - along with the advances in IgA nephropathy therapeutics, which will likely find use in other glomerular diseases, discussed above.
On the other hand, many of you might also point out that dialysis units are not closing down (though in some small towns they are). The incidence count shows why this isn’t happening. In fact, the incident counts have steadily gone up despite the decrease in rates, as the population has grown and aged. But here too, there is reason for hope - see figure 1.1 for counts below. The numbers seem to have plateaued. Has the decrease in rate finally caught up to stabilize the count? Not so fast. This has surely contributed - but something else also happened in 2020. A lot of ESRD patients died of COVID (see figure 21.10 for prevalence count changes) - but how would that change incidence counts? COVID did not just kill dialysis patients. Of the 7 million plus people (source: Our World in Data) who have died of COVID to date, many were those who would have gone on to develop dialysis-dependent renal failure by now. Patients who were elderly, had diabetes, and had advanced CKD had some of the highest mortality rates associated with COVID. This list also, obviously, has significant overlap with patients at risk of ESRD. Hence, this decrease may be an artifact of the vulnerable being removed from the at-risk population. Only time will tell what will happen over the next few decades, once the COVID smoke clears. Regardless of how we interpret this data, we should do our part by implementing evidence-based therapies actively and enthusiastically in every individual with CKD. Let’s see if we can really, actively bend the curve in the next decade.
If you want to revisit previous years’ top nephrology stories, take a look 👇